Intermediate conductance calcium-activated potassium channel (KCa3.1) in cancer: Emerging roles and therapeutic potentials
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
The KCa3.1 channel (also known as the KCNN4, IK1, or SK4 channel) is an intermediate-conductance calcium-activated potassium channel that regulates the membrane potential and maintains calcium homeostasis. Recently, KCa3.1 channels have attracted increasing attention because of their diverse roles in various types of cancers. In cancer cells, KCa3.1 channels regulate key processes, including cell proliferation, cell cycle, migration, invasion, tumor microenvironments, and therapy resistance. In addition, abnormal KCa3.1 expression in cancers is utilized to distinguish between tumor and normal tissues, classify cancer stages, and predict patient survival outcomes. This review comprehensively examines the current understanding of the contribution of KCa3.1 channels to tumor formation, metastasis, and its mechanisms. We evaluated the potential of KCa3.1 as a biomarker for cancer diagnosis and prognosis. Finally, we discuss the advances and challenges of applying KCa3.1 modulators in cancer treatment and propose approaches to overcome these obstacles. In summary, this review highlights the importance of this ion channel as a potent therapeutic target and prognostic biomarker of cancer.
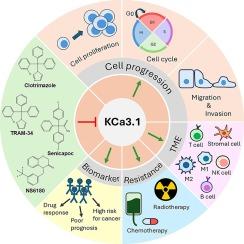
癌症中的中间传导钙激活钾通道(KCa3.1):新的作用和治疗潜力
KCa3.1 通道(又称 KCNN4、IK1 或 SK4 通道)是一种中间传导性钙激活钾通道,可调节膜电位并维持钙平衡。最近,KCa3.1 通道因其在各类癌症中的不同作用而日益受到关注。在癌细胞中,KCa3.1 通道调控着细胞增殖、细胞周期、迁移、侵袭、肿瘤微环境和耐药性等关键过程。此外,癌症中 KCa3.1 的异常表达可用于区分肿瘤和正常组织、划分癌症分期以及预测患者的生存结果。这篇综述全面探讨了目前对 KCa3.1 通道对肿瘤形成、转移的贡献及其机制的理解。我们评估了 KCa3.1 作为癌症诊断和预后生物标志物的潜力。最后,我们讨论了在癌症治疗中应用 KCa3.1 调节剂的进展和挑战,并提出了克服这些障碍的方法。总之,本综述强调了该离子通道作为癌症的有效治疗靶点和预后生物标志物的重要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


