Redirecting the pioneering function of FOXA1 with covalent small molecules
IF 14.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Pioneer transcription factors (TFs) bind to and open closed chromatin, facilitating engagement by other regulatory factors involved in gene activation or repression. Chemical probes are lacking for pioneer TFs, which has hindered their mechanistic investigation in cells. Here, we report the chemical proteomic discovery of electrophilic compounds that stereoselectively and site-specifically bind the pioneer TF forkhead box protein A1 (FOXA1) at a cysteine (C258) within the forkhead DNA-binding domain. We show that these covalent ligands react with FOXA1 in a DNA-dependent manner and rapidly remodel its pioneer activity in prostate cancer cells reflected in redistribution of FOXA1 binding across the genome and directionally correlated changes in chromatin accessibility. Motif analysis supports a mechanism where the ligands relax the canonical DNA-binding preference of FOXA1 by strengthening interactions with suboptimal sequences in predicted proximity to C258. Our findings reveal a striking plasticity underpinning the pioneering function of FOXA1 that can be controlled by small molecules.
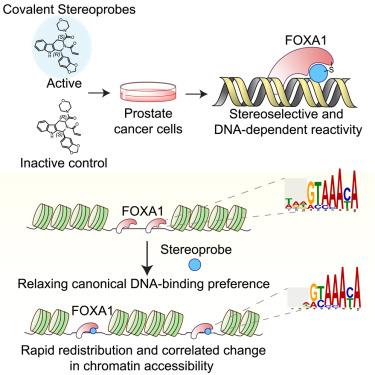
用共价小分子重定向 FOXA1 的先驱功能
先驱转录因子(TFs)与封闭的染色质结合并打开染色质,促进参与基因激活或抑制的其他调控因子的参与。先锋转录因子缺乏化学探针,这阻碍了它们在细胞中的机理研究。在这里,我们报告了化学蛋白质组学发现的亲电化合物,它们能在叉头 DNA 结合域内的半胱氨酸(C258)上立体选择性地结合先锋 TF 叉头盒蛋白 A1(FOXA1)。我们的研究表明,这些共价配体以一种 DNA 依赖性方式与 FOXA1 发生反应,并迅速重塑其在前列腺癌细胞中的先锋活性,这反映在 FOXA1 结合在整个基因组中的重新分布以及染色质可及性中方向相关的变化。基元分析支持一种机制,即配体通过加强与预测的靠近 C258 的次优序列的相互作用来放松 FOXA1 的典型 DNA 结合偏好。我们的研究结果揭示了 FOXA1 先驱功能所具有的惊人的可塑性,这种功能可以由小分子控制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Cell
生物-生化与分子生物学
CiteScore
26.00
自引率
3.80%
发文量
389
审稿时长
1 months
期刊介绍:
Molecular Cell is a companion to Cell, the leading journal of biology and the highest-impact journal in the world. Launched in December 1997 and published monthly. Molecular Cell is dedicated to publishing cutting-edge research in molecular biology, focusing on fundamental cellular processes. The journal encompasses a wide range of topics, including DNA replication, recombination, and repair; Chromatin biology and genome organization; Transcription; RNA processing and decay; Non-coding RNA function; Translation; Protein folding, modification, and quality control; Signal transduction pathways; Cell cycle and checkpoints; Cell death; Autophagy; Metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


