Folate-Functionalized Chitosan-PLGA Nanoparticles: A Novel approach for targeted osthole delivery in pancreatic cancer
IF 2.5
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Efficient drug delivery systems targeting cancer cells are crucial for enhancing cancer therapy. In this study, we developed PLGA nanoparticles coated with folate-conjugated chitosan (osthole-PLGA-NPs/CS-FA) to deliver osthole to cancer cells and investigated its inhibitory and molecular signaling mechanisms in the PANC-1 pancreatic cancer cell line. Field emission scanning electron microscopy (FESEM) revealed that osthole-PLGA-NPs/CS-FA had a spherical structure with a uniform size distribution. Dynamic light scattering (DLS) analysis showed an average size of 171.76 nm, a dispersion index 0.26, and a surface charge of + 33.08 mV, indicating stability and uniform dispersion. Fourier-transform infrared (FTIR) spectrum analysis confirmed the successful incorporation of osthole into the PLGA nanoparticles, with an encapsulation efficiency of 93.12 %. These physicochemical properties suggest efficient cellular uptake and targeted delivery. The antioxidant potential of osthole-PLGA-NPs/CS-FA was evaluated using the ABTS assay, showing concentration-dependent inhibition of free radicals with an IC50 value of 172.95 μg/mL. The anticancer properties were assessed using the MTT assay, demonstrating a significant and concentration-dependent cytotoxic effect on PANC-1 cells (IC50 = 31.2 μg/mL) with minimal impact on normal human foreskin fibroblast (HFF) cells. DAPI staining and flow cytometry analyses confirmed a concentration-dependent increase in apoptosis in PANC-1 cells. The nanoparticles induced upregulation of Bax and downregulation of Bcl2, indicating activation of the intrinsic mitochondrial apoptotic pathway. The anti-angiogenic activity of osthole-PLGA-NPs/CS-FA was evaluated using the chick chorioallantoic membrane (CAM) assay. The results showed significant inhibition of angiogenesis in a concentration-dependent manner, starting at 40 μg/mL and increasing up to 120 μg/mL. In conclusion, osthole-PLGA-NPs/CS-FA nanoparticles exhibit promising potential for targeted pancreatic cancer therapy by enhancing cellular uptake, inducing apoptosis, and inhibiting angiogenesis.
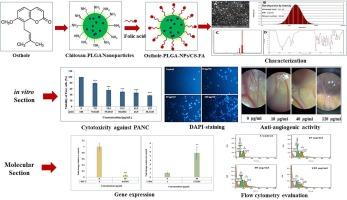
叶酸功能化壳聚糖-PLGA 纳米粒子:胰腺癌靶向递送叶酸的新方法
高效的靶向癌细胞给药系统对于提高癌症治疗效果至关重要。在这项研究中,我们开发了包覆叶酸共轭壳聚糖的 PLGA 纳米颗粒(osthole-PLGA-NPs/CS-FA),用于向癌细胞递送 osthole,并研究了其在 PANC-1 胰腺癌细胞系中的抑制和分子信号转导机制。场发射扫描电子显微镜(FESEM)显示,osthole-PLGA-NPs/CS-FA呈球形结构,大小分布均匀。动态光散射(DLS)分析表明,其平均粒度为 171.76 nm,分散指数为 0.26,表面电荷为 + 33.08 mV,表明其稳定性和分散均匀性。傅立叶变换红外光谱(FTIR)分析证实,奥斯特孔成功地融入了聚乳酸乙二醛(PLGA)纳米颗粒,封装效率高达 93.12%。这些理化特性表明,奥斯特孔能被细胞高效吸收并进行靶向递送。使用 ABTS 法评估了 osthole-PLGA-NPs/CS-FA 的抗氧化潜力,结果表明其抑制自由基的作用与浓度有关,IC50 值为 172.95 μg/mL。使用 MTT 试验评估了其抗癌特性,结果表明它对 PANC-1 细胞具有显著的浓度依赖性细胞毒性作用(IC50 = 31.2 μg/mL),对正常人包皮成纤维细胞(HFF)的影响极小。DAPI 染色和流式细胞仪分析证实,PANC-1 细胞凋亡的增加与浓度有关。纳米颗粒诱导了 Bax 的上调和 Bcl2 的下调,表明线粒体内在凋亡途径被激活。使用小鸡绒毛膜(CAM)试验评估了 osthole-PLGA-NPs/CS-FA 的抗血管生成活性。结果表明,从 40 μg/mL开始,到 120 μg/mL为止,该物质以浓度依赖的方式明显抑制了血管生成。总之,osthole-PLGA-NPs/CS-FA 纳米粒子通过增强细胞摄取、诱导细胞凋亡和抑制血管生成,在胰腺癌靶向治疗中展现出了巨大的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


