Reduced microRNA-744 expression in mast cell-derived exosomes triggers epithelial cell ferroptosis in acute respiratory distress syndrome
IF 10.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Acute respiratory distress syndrome (ARDS) is a critical disorder characterized by immune-related damage to epithelial cells; however, its underlying mechanism remains elusive. This study investigated the effects of alterations in microRNA (miRNA) expression in mast cell-derived exosomes on human bronchial epithelial (HBE) cells and ARDS development in cellular and mouse models challenged with lipopolysaccharide. Lipopolysaccharide-treated mast cell-derived exosomes reduced glutathione peroxidase 4 (GPX4) expression and increased long-chain acyl-CoA synthetase 4 (ACSL4), 15-lipoxygenase (ALOX15), and inflammatory mediator levels in HBE cells. miRNA sequencing revealed a reduction in mast cell-derived exosomal miR-744 levels, which was associated with the regulation of ACSL4, ALOX15, and GPX4 expression. This downregulation of exosomal miR-744 expression reduced miR-744 levels and promoted ferroptosis in HBE cells, whereas the experimental upregulation of miR-744 reversed these adverse effects. Down-regulation of miR-744 induced the expression of markers for ferroptosis and inflammation in HBE cells and promoted pulmonary ferroptosis, inflammation, and injury in LPS-stimulated mice. In vivo, treatment with ACSL4, ALOX15, and GPX4 inhibitors mitigated these effects, and experimental miR-744 expression rescued the lipopolysaccharide-induced changes in HBE cells and mouse lungs. Notably, miR-744 levels were reduced in the plasma and exosomes of patients with ARDS. We concluded that decreased mast cell-derived exosomal miR-744 levels trigger epithelial cell ferroptosis, promoting lung inflammation and damage in ARDS. This study provides new mechanistic insights into the development and sustained pulmonary damage associated with ARDS and highlights potential therapeutic strategies.
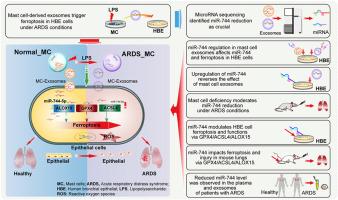
肥大细胞衍生的外泌体中的 microRNA-744 表达减少会引发急性呼吸窘迫综合征中上皮细胞的铁变态反应。
急性呼吸窘迫综合征(ARDS)是一种危重疾病,其特征是上皮细胞受到免疫相关的损伤;然而,其潜在的机制仍然难以捉摸。本研究调查了肥大细胞衍生的外泌体中微量RNA(miRNA)表达的改变对人支气管上皮(HBE)细胞的影响,以及细胞和小鼠模型在脂多糖挑战下发生ARDS的情况。经脂多糖处理的肥大细胞源性外泌体降低了谷胱甘肽过氧化物酶4(GPX4)的表达,并增加了HBE细胞中长链酰基-CoA合成酶4(ACSL4)、15-脂氧合酶(ALOX15)和炎症介质的水平。miRNA测序显示肥大细胞源性外泌体miR-744水平的降低与ACSL4、ALOX15和GPX4表达的调节有关。这种外泌体 miR-744 表达的下调降低了 miR-744 的水平,并促进了 HBE 细胞中的铁变态反应,而实验性的 miR-744 上调则逆转了这些不利影响。下调 miR-744 可诱导 HBE 细胞中铁细胞减少症和炎症标志物的表达,并促进 LPS 刺激小鼠肺铁细胞减少症、炎症和损伤。在体内,用 ACSL4、ALOX15 和 GPX4 抑制剂治疗可减轻这些影响,而实验性 miR-744 表达可挽救脂多糖诱导的 HBE 细胞和小鼠肺部变化。值得注意的是,ARDS 患者血浆和外泌体中的 miR-744 水平降低了。我们的结论是,肥大细胞衍生的外泌体 miR-744 水平降低会触发上皮细胞铁蛋白沉积,促进 ARDS 中的肺部炎症和损伤。这项研究为了解与 ARDS 相关的肺损伤的发展和持续提供了新的机理认识,并强调了潜在的治疗策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Redox Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-
CiteScore
19.90
自引率
3.50%
发文量
318
审稿时长
25 days
期刊介绍:
Redox Biology is the official journal of the Society for Redox Biology and Medicine and the Society for Free Radical Research-Europe. It is also affiliated with the International Society for Free Radical Research (SFRRI). This journal serves as a platform for publishing pioneering research, innovative methods, and comprehensive review articles in the field of redox biology, encompassing both health and disease.
Redox Biology welcomes various forms of contributions, including research articles (short or full communications), methods, mini-reviews, and commentaries. Through its diverse range of published content, Redox Biology aims to foster advancements and insights in the understanding of redox biology and its implications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


