Ligand engineering regulates the electronic structure of Ni-N-C sites to promote electrocatalytic acetylene semi-hydrogenation
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Compared with traditional thermal catalytic hydrogenation technology, electrocatalytic acetylene semi-hydrogenation (EASH) is a green and environmentally friendly method. EASH utilizes renewable electricity under normal temperature and pressure conditions, and uses water as a hydrogen source to electrocatalytically semi-hydrogenate acetylene to ethylene. Ni-based single-atom catalysts (SACs) have shown good application prospects in EASH, but there is still much room for optimization. The axial coordination regulation of the active center is a potential means to improve its activity. In this work, the activity and selectivity of NiN4 SACs with 14 axial ligands (X) for EASH were systematically studied via density functional theory (DFT). First, the binding energy and ab initio molecular dynamics simulation results show that all the NiN4-X can exist stably. Second, the Gibbs free energy diagram shows that the activation and adsorption of C2H2 is the rate-determining step of the reaction. The introduction of the –ONO ligand enhances the activation and adsorption of C2H2 by NiN4, which is beneficial to the occurrence of EASH and inhibits the side reactions of hydrogen evolution. The two parameters and can be used as characteristic descriptors to predict the activity and selectivity of EASH. Finally, the catalytic activity mechanism was explained from the perspective of electrons and orbitals, and it was found that the orbital played a leading role in the axial ligand regulation of C2H2 catalytic activity. This work provides a new method for the development of efficient EASH catalysts.
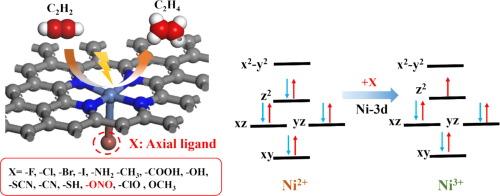
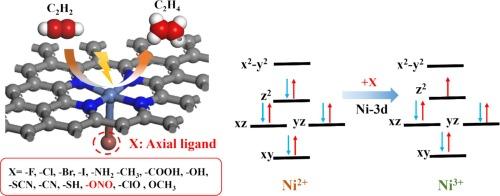
配体工程调节 Ni-N-C 位点的电子结构,促进电催化乙炔半加氢反应
与传统的热催化加氢技术相比,电催化乙炔半加氢(EASH)是一种绿色环保的方法。EASH 在常温常压条件下利用可再生电力,以水为氢源,通过电催化将乙炔半加氢为乙烯。镍基单原子催化剂(SAC)在 EASH 中具有良好的应用前景,但仍有很大的优化空间。活性中心的轴向配位调节是提高其活性的潜在手段。本研究通过密度泛函理论(DFT)系统研究了含有 14 个轴向配体(X)的 NiN4 SACs 在 EASH 中的活性和选择性。首先,结合能和 ab initio 分子动力学模拟结果表明,所有 NiN4-X 都能稳定存在。其次,吉布斯自由能图表明,C2H2 的活化和吸附是反应的速率决定步骤。-ONO 配体的引入增强了 NiN4 对 C2H2 的活化和吸附,有利于 EASH 的发生,并抑制了氢气演化的副反应。ΔG∗CHCH和ΔG∗CH2CH3这两个参数可作为特征描述因子来预测EASH的活性和选择性。最后,从电子和轨道的角度解释了催化活性机理,发现 dz2 轨道在轴向配体调节 C2H2 催化活性中起主导作用。这项工作为开发高效的 EASH 催化剂提供了一种新方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


