Air-stable, well-defined palladium–BIAN–NHC chloro dimer: Highly efficient N-Heterocyclic carbene (NHC) catalyst platform for Buchwald–Hartwig C–N cross-coupling reactions
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Buchwald-Hartwig amination has become the fundamental method for constructing molecular architectures throughout chemical research, including the synthesis of pharmaceutical agents, natural products, fine chemicals, and advanced materials. Herein, we report air-stable, well-defined palladium–BIAN–NHC chloro dimer, [Pd(BIAN–NHC)(μ-Cl)Cl]2, for Buchwald-Hartwig C–N cross-coupling reactions of aryl halides. This rapidly activating catalyst framework merges the reactive properties of palladium chloro dimers, [Pd(NHC)(μ-Cl)Cl]2, with the structural features of acenaphthoimidazol-2-ylidenes. [Pd(BIAN–NHC)(μ-Cl)Cl]2 is the most reactive Pd(II)–NHC precatalyst to date, undergoing fast activation under both inert atmosphere and aerobic conditions. The catalyst shows an excellent reactivity in Buchwald-Hartwig amination of aryl halides (59 examples), including challenging substrates, diamination and direct functionalization of pharmaceuticals. The steric protection enables high reactivity under both inert atmosphere and aerobic conditions. [Pd(BIAN–IPr)(μ-Cl)Cl]2 should be routinely utilized for the synthesis of C–N bonds to make valuable amines, where it replaces the most commonly deployed at present IPr (IPr = 1,3-bis(2,6-isopropyl)imidazol-2-ylidene).
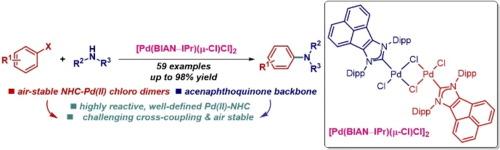
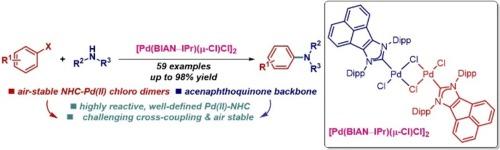
空气稳定、定义明确的钯-BIAN-NHC 氯二聚体:用于布赫瓦尔德-哈特维格 C-N 交叉偶联反应的高效 N-杂环碳烯 (NHC) 催化剂平台
布赫瓦尔德-哈特维格胺化法已成为整个化学研究领域构建分子结构的基本方法,包括合成药剂、天然产物、精细化学品和先进材料。在此,我们报告了空气稳定、定义明确的钯-BIAN-NHC 氯二聚体 [Pd(BIAN-NHC)(μ-Cl)Cl]2,用于芳基卤化物的布氏-哈氏 C-N 交叉偶联反应。这种快速活化催化剂框架融合了钯氯二聚体[Pd(NHC)(μ-Cl)Cl]2 的反应特性和苊基咪唑-2-亚基的结构特征。[Pd(BIAN-NHC)(μ-Cl)Cl]2是迄今为止活性最高的钯(II)-NHC前催化剂,在惰性气氛和有氧条件下都能快速活化。该催化剂在芳基卤化物的布赫瓦尔德-哈特维格胺化反应(59 个实例)中表现出极佳的反应活性,包括具有挑战性的底物、二胺化和药物的直接官能化。立体保护使其在惰性气氛和有氧条件下都具有很高的反应活性。[Pd(BIAN-IPr)(μ-Cl)Cl]2应常规用于合成C-N键,以制造有价值的胺,它可以取代目前最常用的IPr(IPr = 1,3-双(2,6-异丙基)咪唑-2-亚基)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


