Dynamic structural remodeling of LINC01956 enhances temozolomide resistance in MGMT-methylated glioblastoma
IF 15.8
1区 医学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
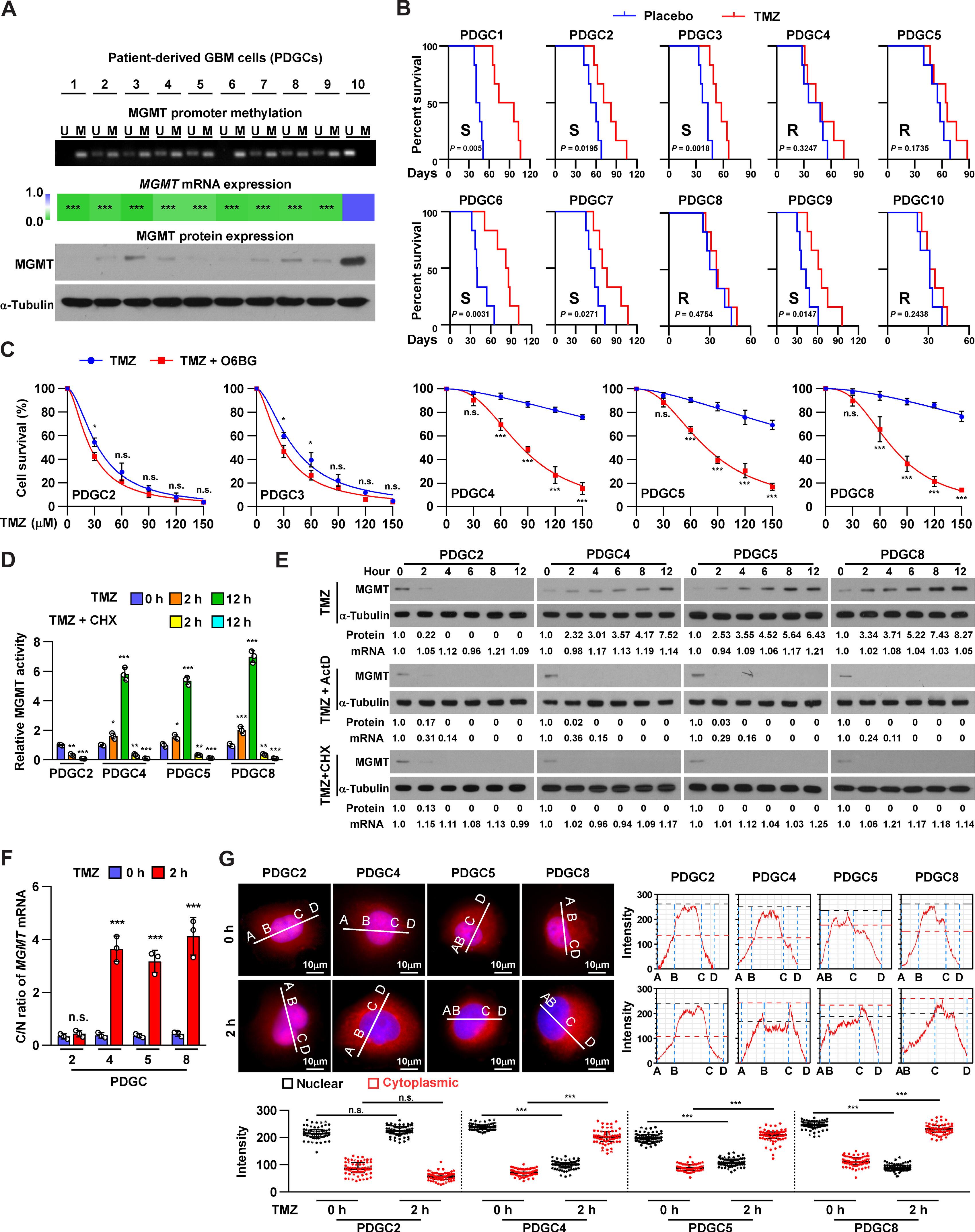
The mechanisms underlying stimuli-induced dynamic structural remodeling of RNAs for the maintenance of cellular physiological function and survival remain unclear. Here, we showed that in MGMT promoter–methylated glioblastoma (GBM), the RNA helicase DEAD-box helicase 46 (DDX46) is phosphorylated by temozolomide (TMZ)–activated checkpoint kinase 1 (CHK1), resulting in a dense-to-loose conformational change and an increase in DDX46 helicase activity. DDX46-mediated tertiary structural remodeling of LINC01956 exposes the binding motifs of LINC01956 to the 3′ untranslated region of O6-methylguanine DNA methyltransferase (MGMT). This accelerates recruitment of MGMT mRNA to the RNA export machinery and transportation of MGMT mRNA from the nucleus to the cytoplasm, leading to increased MGMT abundance and TMZ resistance. Using patient-derived xenograft (PDX) and tumor organoid models, we found that treatment with the CHK1 inhibitor SRA737abolishes TMZ-induced structural remodeling of LINC01956 and subsequent MGMT up-regulation, resensitizing TMZ-resistant MGMT promoter–methylated GBM to TMZ. In conclusion, these findings highlight a mechanism underlying temozolomide-induced RNA structural remodeling and may represent a potential therapeutic strategy for patients with TMZ-resistant MGMT promoter–methylated GBM.
LINC01956 的动态结构重塑增强了 MGMT 甲基化胶质母细胞瘤对替莫唑胺的耐药性
刺激诱导 RNA 动态结构重塑以维持细胞生理功能和存活的机制仍不清楚。在这里,我们发现在MGMT启动子甲基化的胶质母细胞瘤(GBM)中,RNA螺旋酶DEAD-box helicase 46(DDX46)被替莫唑胺(TMZ)激活的检查点激酶1(CHK1)磷酸化,导致致密到松弛的构象变化以及DDX46螺旋酶活性的增加。DDX46 介导的 LINC01956 三级结构重塑使 LINC01956 与 O 6 -甲基鸟嘌呤 DNA 甲基转移酶(MGMT)3′非翻译区的结合基序暴露出来。这加速了 MGMT mRNA 与 RNA 导出机制的结合,并将 MGMT mRNA 从细胞核运输到细胞质,从而导致 MGMT 丰度增加和 TMZ 抗性。利用患者衍生异种移植(PDX)和肿瘤类器官模型,我们发现用 CHK1 抑制剂 SRA737 治疗可消除 TMZ 诱导的 LINC01956 结构重塑和随后的 MGMT 上调,使 TMZ 耐药的 MGMT 启动子甲基化 GBM 对 TMZ 再敏感。总之,这些发现突显了替莫唑胺诱导 RNA 结构重塑的机制,可能是治疗 TMZ 耐药 MGMT 启动子甲基化 GBM 患者的一种潜在策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Translational Medicine
CELL BIOLOGY-MEDICINE, RESEARCH & EXPERIMENTAL
CiteScore
26.70
自引率
1.20%
发文量
309
审稿时长
1.7 months
期刊介绍:
Science Translational Medicine is an online journal that focuses on publishing research at the intersection of science, engineering, and medicine. The goal of the journal is to promote human health by providing a platform for researchers from various disciplines to communicate their latest advancements in biomedical, translational, and clinical research.
The journal aims to address the slow translation of scientific knowledge into effective treatments and health measures. It publishes articles that fill the knowledge gaps between preclinical research and medical applications, with a focus on accelerating the translation of knowledge into new ways of preventing, diagnosing, and treating human diseases.
The scope of Science Translational Medicine includes various areas such as cardiovascular disease, immunology/vaccines, metabolism/diabetes/obesity, neuroscience/neurology/psychiatry, cancer, infectious diseases, policy, behavior, bioengineering, chemical genomics/drug discovery, imaging, applied physical sciences, medical nanotechnology, drug delivery, biomarkers, gene therapy/regenerative medicine, toxicology and pharmacokinetics, data mining, cell culture, animal and human studies, medical informatics, and other interdisciplinary approaches to medicine.
The target audience of the journal includes researchers and management in academia, government, and the biotechnology and pharmaceutical industries. It is also relevant to physician scientists, regulators, policy makers, investors, business developers, and funding agencies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


