Acyl-CoA long-chain synthetase 1 (ACSL1) protects the endometrium from excess palmitic acid stress during decidualization
IF 4.4
2区 生物学
Q2 CELL BIOLOGY
引用次数: 0
Abstract
Endometrial receptivity relies on the functional and morphological change of endometrium stromal cells (EnSCs) and epithelial cells in the secretory phase. Decidualization of ESCs and transitions in endometrium epithelial cells are crucial for successful uterine implantation and maintaining pregnancy. Accumulated data have demonstrated that decidualization is tightly coordinated by lipid metabolism. However, the lipidomic change and regulatory mechanism in uterine decidualization are still unknown. Our study showed that endometrium stromal cells and decidual stromal cells had different lipidomic profiles. Acyl-CoA long-chain synthetase 1 (ACSL1) which converts fatty acids to acyl-CoA expression was strongly elevated during decidualization. ACSL1 knockdown inhibited stromal-to-decidual cell transition and decreased the decidualization markers prolactin and Insulin-like growth factor-binding protein-1 (IGFBP1) expression through the AKT pathway. Lipid uptake was upregulated in stromal cells while lipid droplet accumulation was downregulated during decidualization. Meanwhile, silencing of ACSL1 led to impaired spare respiratory capacity, and downregulation of TFAM expression, indicating robust lipid metabolism. While palmitic acid addition impeded decidualization, overexpression of ACSL1 could partially reverse its effect. ACSL inhibitor Triacsin C significantly impeded decidualization in a three-dimensional coculture model consisting of endometrial stromal cells and epithelial cells. Knockdown of ACSL1 in stromal cells decreased the expression of the decidualization markers PAEP and SPP1 in epithelial cells. Collectively, ACSL1 is essential for uterine decidualization and protects stromal cells from excess palmitic acid stress.
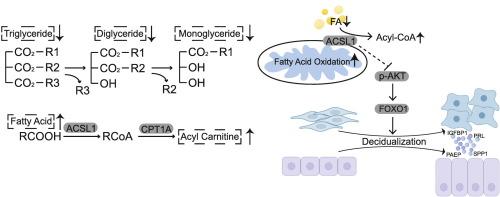
Acyl-CoA long-chain synthetase 1(ACSL1)可在蜕膜化过程中保护子宫内膜免受过量棕榈酸的压力。
子宫内膜的受孕能力取决于分泌期子宫内膜基质细胞(ESCs)和上皮细胞的功能和形态变化。胚胎干细胞的蜕膜化和子宫内膜上皮细胞的转变是成功植入子宫和维持妊娠的关键。积累的数据表明,蜕膜化与脂质代谢密切相关。然而,子宫蜕膜化过程中的脂质组变化和调控机制仍然未知。我们的研究表明,子宫内膜基质细胞和蜕膜基质细胞具有不同的脂质组学特征。在蜕膜化过程中,将脂肪酸转化为酰基-CoA的酰基-CoA长链合成酶1(ACSL1)的表达强烈升高。敲除ACSL1抑制了基质细胞向蜕膜细胞的转化,并通过AKT途径降低了蜕膜标志物催乳素和胰岛素样生长因子结合蛋白-1(IGFBP1)的表达。在蜕膜化过程中,基质细胞的脂质摄取上调,而脂滴积累下调。同时,沉默ACSL1会导致备用呼吸能力受损和TFAM表达下调,表明脂质代谢旺盛。虽然棕榈酸的添加阻碍了蜕膜化,但过表达 ACSL1 可部分逆转其影响。在由子宫内膜基质细胞和上皮细胞组成的三维共培养模型中,ACSL抑制剂Triacsin C明显阻碍了蜕膜化。在基质细胞中敲除 ACSL1 会降低上皮细胞中蜕膜化标记 PAEP 和 SPP1 的表达。总而言之,ACSL1对子宫蜕膜化至关重要,它能保护基质细胞免受过量棕榈酸压力的影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cellular signalling
生物-细胞生物学
CiteScore
8.40
自引率
0.00%
发文量
250
审稿时长
27 days
期刊介绍:
Cellular Signalling publishes original research describing fundamental and clinical findings on the mechanisms, actions and structural components of cellular signalling systems in vitro and in vivo.
Cellular Signalling aims at full length research papers defining signalling systems ranging from microorganisms to cells, tissues and higher organisms.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


