Scaling Laws for Protein Folding under Confinement
IF 4.8
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
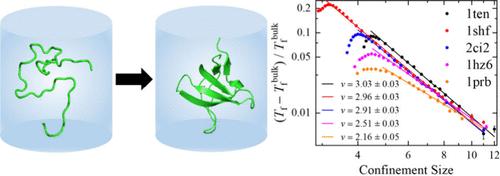
Spatial confinement significantly affects protein folding. Without the confinement provided by chaperones, many proteins cannot fold correctly. However, the quantitative effect of confinement on protein folding remains elusive. In this study, we observed scaling laws between the variation in folding transition temperature and the size of confinement, (Tf – Tfbulk)/Tfbulk ∼ L–ν. The scaling exponent v is significantly influenced by both the protein’s topology and folding cooperativity. Specifically, for a given protein, v can decrease as the folding cooperativity of the model increases, primarily due to the heightened sensitivity of the unfolded state energy to changes in cage size. For proteins with diverse topologies, variations in topological complexity influence scaling exponents in multiple ways. Notably, v exhibits a clear positive correlation with contact order and the proportion of nonlocal contacts, as this complexity significantly enhances the sensitivity of entropy loss in the unfolded state. Furthermore, we developed a novel scaling argument yielding 5/3 ≤ ν ≤ 10/3, consistent with the simulation results.
封闭条件下蛋白质折叠的缩放定律
空间限制对蛋白质折叠有重大影响。如果没有伴侣提供的限制,许多蛋白质就无法正确折叠。然而,限制对蛋白质折叠的定量影响仍然难以捉摸。在这项研究中,我们观察到折叠转变温度的变化与封闭大小之间的缩放规律,即 (Tf - Tfbulk)/Tfbulk ∼ L-ν。缩放指数 v 受到蛋白质拓扑结构和折叠合作性的显著影响。具体来说,对于给定的蛋白质,v 会随着模型折叠合作性的增加而减小,这主要是由于展开态能量对笼子大小变化的敏感性增加。对于具有不同拓扑结构的蛋白质,拓扑复杂性的变化会以多种方式影响缩放指数。值得注意的是,v 与接触顺序和非局部接触的比例呈现出明显的正相关,因为这种复杂性显著提高了展开态熵损失的敏感性。此外,我们还提出了一个新的缩放参数,即 5/3 ≤ ν ≤ 10/3,与模拟结果一致。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry Letters
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
9.60
自引率
7.00%
发文量
1519
审稿时长
1.6 months
期刊介绍:
The Journal of Physical Chemistry (JPC) Letters is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, chemical physicists, physicists, material scientists, and engineers. An important criterion for acceptance is that the paper reports a significant scientific advance and/or physical insight such that rapid publication is essential. Two issues of JPC Letters are published each month.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


