Development of a Chitin-Based Purification System Utilizing Chitin-Binding Domain and Tobacco Etch Virus Protease Cleavage for Efficient Recombinant Protein Recovery
IF 5.7
1区 农林科学
Q1 AGRICULTURE, MULTIDISCIPLINARY
引用次数: 0
Abstract
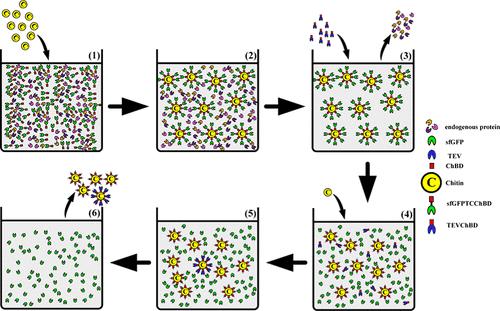
This study aims to develop an efficient chitin-based purification system, leveraging a novel design where the target proteins, superfolding green fluorescent protein (sfGFP) and Thermus antranikianii trehalose synthase (TaTS), fused with a chitin-binding domain (ChBD) from Bacillus circulans WL-12 chitinase A1 and a tobacco etch virus protease (TEVp) cleavage site. This configuration allows for the effective immobilization of the target proteins on chitin beads, facilitating the removal of endogenous proteins. A mutant TEVp, H-TEVS219V-ChBD, fused with the His-tag and ChBD, is employed to cleave the target proteins from the chitin beads specifically. Subsequently, fresh chitin beads are added for adsorption to remove H-TEVS219V-ChBD in the solution, thereby significantly improving the purity of the target protein. Our results confirm that this system can efficiently and specifically purify and recover sfGFP and TaTS, achieving electrophoretic-grade purity exceeding 90%. This system holds significant potential for industrial production and other applications.
开发基于几丁质的纯化系统,利用几丁质结合域和烟草蚀刻病毒蛋白酶裂解作用高效回收重组蛋白
本研究旨在开发一种高效的基于几丁质的纯化系统,利用一种新颖的设计,将目标蛋白--超折叠绿色荧光蛋白(sfGFP)和Thermus antranikianii三卤糖合酶(TaTS)--与来自环状芽孢杆菌WL-12几丁质酶A1的几丁质结合域(ChBD)和烟草蚀病毒蛋白酶(TEVp)裂解位点融合在一起。这种结构可将目标蛋白质有效固定在几丁质珠上,便于去除内源性蛋白质。使用融合了 His 标记和 ChBD 的突变 TEVp H-TEVS219V-ChBD,可特异性地从几丁质珠上裂解目标蛋白质。随后,加入新的几丁质珠进行吸附,以去除溶液中的 H-TEVS219V-ChBD,从而显著提高目标蛋白质的纯度。我们的研究结果证实,该系统能高效、特异地纯化和回收 sfGFP 和 TaTS,电泳纯度超过 90%。该系统在工业生产和其他应用方面具有巨大潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.90
自引率
8.20%
发文量
1375
审稿时长
2.3 months
期刊介绍:
The Journal of Agricultural and Food Chemistry publishes high-quality, cutting edge original research representing complete studies and research advances dealing with the chemistry and biochemistry of agriculture and food. The Journal also encourages papers with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


