Advanced electrochemical oxidation of EDTA-Ni via cobalt single-atom catalysts: Exploring indirect persulfate activation pathways
IF 5.7
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
This study explores an innovative electrochemical strategy for the removal of the highly stable and toxic EDTA-Ni complex found in electroplating wastewater. Utilizing a cobalt-based single-atom catalyst (Co-NC) in an electro-enhanced system, we achieved significant activation of peroxydisulfate (PDS) for effective degradation of EDTA-Ni. Under optimal conditions of 40 mA current density and a pH range of 3–7, more than 97% of EDTA-Ni (1 mM) was successfully degraded within 90 min. Through detailed electrochemical experiments, we identified that atomic hydrogen (H*) played a crucial role in the indirect activation of PDS, facilitating the formation of reactive sulfate radicals (·SO4-). Computational analysis using density functional theory (DFT) confirmed that the H*-mediated reduction pathway had a notably low energy barrier (ΔGbs = 0.51 eV), making it the dominant activation mechanism. Gas chromatography-mass spectrometry (GC–MS) further revealed the primary degradation intermediates, providing insights into the breakdown process of EDTA-Ni. This research underscores the potential of Co-NC catalyst as a highly effective catalyst for treating persistent heavy metal complexes in advanced oxidation systems.
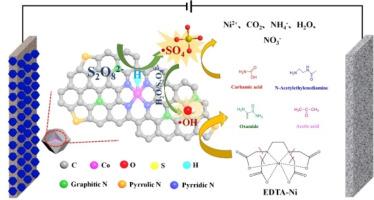
通过钴单原子催化剂实现 EDTA-Ni 的高级电化学氧化:探索间接过硫酸盐活化途径
本研究探索了一种创新的电化学策略,用于去除电镀废水中高度稳定且有毒的 EDTA-Ni 复合物。利用电增强系统中的钴基单原子催化剂(Co-NC),我们实现了过硫酸盐(PDS)的显著活化,从而有效降解 EDTA-Ni。在电流密度为 40 mA、pH 值为 3-7 的最佳条件下,超过 97% 的 EDTA-Ni(1 mM)在 90 分钟内被成功降解。通过详细的电化学实验,我们发现原子氢(H*)在 PDS 的间接活化过程中发挥了关键作用,促进了活性硫酸根自由基(-SO4-)的形成。利用密度泛函理论(DFT)进行的计算分析证实,氢*介导的还原途径具有明显较低的能垒(ΔGbs = 0.51 eV),使其成为主要的活化机制。气相色谱-质谱分析法(GC-MS)进一步揭示了主要降解中间产物,为了解乙二胺四乙酸二钠的分解过程提供了线索。这项研究强调了 Co-NC 催化剂作为一种高效催化剂在高级氧化系统中处理持久性重金属络合物的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Surfaces and Interfaces
Chemistry-General Chemistry
CiteScore
8.50
自引率
6.50%
发文量
753
审稿时长
35 days
期刊介绍:
The aim of the journal is to provide a respectful outlet for ''sound science'' papers in all research areas on surfaces and interfaces. We define sound science papers as papers that describe new and well-executed research, but that do not necessarily provide brand new insights or are merely a description of research results.
Surfaces and Interfaces publishes research papers in all fields of surface science which may not always find the right home on first submission to our Elsevier sister journals (Applied Surface, Surface and Coatings Technology, Thin Solid Films)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


