Mechanism of Dual-Site Recognition in a Classic DNA Aptamer
IF 5.6
2区 化学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Nucleic acid aptamers possess unique advantages in specific recognition. However, the lack of in-depth investigation into their dynamic recognition mechanisms has restricted their rational design and potential applications in fields such as biosensing and targeted therapy. We herein utilized enhanced sampling molecular dynamics to address affinities of adenosine monophosphate (AMP) to the dual binding sites in the DNA aptamer, focusing on the dynamic recognition mechanism and pathways. The present results indicate that in addition to the widely known intermolecular interactions, inequivalence of chemical environments of the two binding sites leads to slightly higher stability of AMP binding to the site proximal to the aptamer terminus. In the presence of two AMPs captured by the two sites, each binding free energy is enhanced. In particular, an additional hydrogen bond of AMP to A10 is introduced in the dual-site binding complex, which increases the binding energy from −4.25 ± 0.47 to −9.48 ± 0.33 kcal mol–1 in the site close to the loop. For the dual-site recognition process, the free energy landscape and minimum free energy pathway calculations elucidate the crucial role of electrostatic interactions between the AMP phosphate groups and Na+ ions in positively cooperative binding mechanisms.
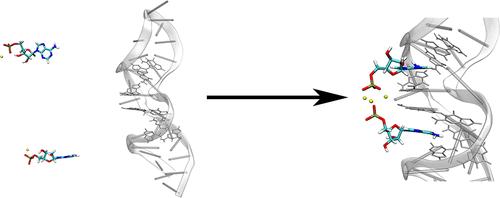
经典 DNA 七聚体的双位点识别机制
核酸适配体在特异性识别方面具有独特的优势。然而,由于缺乏对其动态识别机制的深入研究,限制了其在生物传感和靶向治疗等领域的合理设计和潜在应用。在此,我们利用增强采样分子动力学方法研究了单磷酸腺苷(AMP)与 DNA 合体中双重结合位点的亲和力,重点研究了其动态识别机制和途径。目前的研究结果表明,除了广为人知的分子间相互作用外,两个结合位点化学环境的不对等导致 AMP 与接近适配体末端的位点结合的稳定性略高。在两个结合位点捕获两个 AMP 的情况下,每个结合位点的自由能都会增强。特别是,在双位点结合复合物中,AMP 与 A10 之间引入了一个额外的氢键,这使得靠近环的位点的结合能从-4.25 ± 0.47 kcal mol-1 增加到-9.48 ± 0.33 kcal mol-1。对于双位点识别过程,自由能景观和最小自由能路径计算阐明了 AMP 磷酸基团和 Na+ 离子之间的静电相互作用在正合作结合机制中的关键作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.80
自引率
10.70%
发文量
529
审稿时长
1.4 months
期刊介绍:
The Journal of Chemical Information and Modeling publishes papers reporting new methodology and/or important applications in the fields of chemical informatics and molecular modeling. Specific topics include the representation and computer-based searching of chemical databases, molecular modeling, computer-aided molecular design of new materials, catalysts, or ligands, development of new computational methods or efficient algorithms for chemical software, and biopharmaceutical chemistry including analyses of biological activity and other issues related to drug discovery.
Astute chemists, computer scientists, and information specialists look to this monthly’s insightful research studies, programming innovations, and software reviews to keep current with advances in this integral, multidisciplinary field.
As a subscriber you’ll stay abreast of database search systems, use of graph theory in chemical problems, substructure search systems, pattern recognition and clustering, analysis of chemical and physical data, molecular modeling, graphics and natural language interfaces, bibliometric and citation analysis, and synthesis design and reactions databases.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


