Nickel-Catalyzed Enantioselective Alkylation of Primary Phosphines
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Functional molecules derived from stereogenic phosphorus centers have important applications in the discovery of drugs and agrochemicals. They are also widely utilized as chiral ligands or organocatalysts for diverse asymmetric transformations. However, access to P-stereogenic motifs has always been regarded as a highly challenging yet desirable goal in organic synthesis. The development of general and practical methods for the stereoselective construction of synthetically versatile P(III)-stereogenic phosphines is particularly appealing but remains elusive. Herein, we describe a nickel-catalyzed asymmetric alkylation of primary phosphines with alkyl halides for the synthesis of P-stereogenic secondary phosphine-boranes with high enantioselectivity and broad substrate scope. The resulting optically active secondary phosphine-boranes allow for further stereospecific transformations, thereby establishing a modular and efficient platform for the diversity-oriented construction of P-stereogenic phosphine compounds.
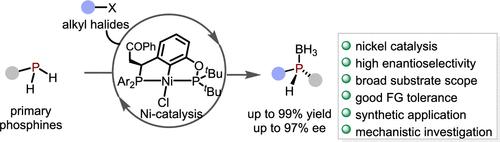
镍催化伯胺膦的对映选择性烷基化反应
源自立体磷中心的功能分子在药物和农用化学品的研发中有着重要的应用。它们还被广泛用作手性配体或有机催化剂,用于各种不对称转化。然而,在有机合成中,获得 P-stereogenic motifs 一直被认为是一个极具挑战性但又令人向往的目标。开发通用而实用的方法来立体选择性地构建合成多用途的 P(III) 立体源膦尤其具有吸引力,但仍然难以实现。在此,我们介绍了一种镍催化的伯膦与烷基卤化物的不对称烷基化反应,用于合成具有高对映选择性和广泛底物范围的 P-stereogenic 仲膦硼烷。由此得到的光学活性仲膦硼烷可用于进一步的立体特异性转化,从而为以多样性为导向构建 P-stereogenic 磷化合物建立了一个模块化的高效平台。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


