Understanding Boron Chemistry as the Surface Modification and Electrolyte Additive for Co‐Free Lithium‐Rich Layered Oxide
IF 24.4
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
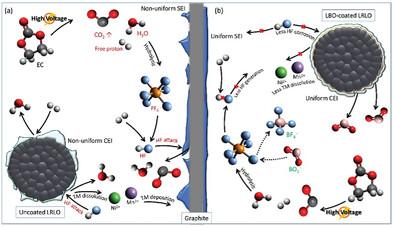
Lithium‐rich layered oxide (LRLO) stands out as a highly promising cathode material for the next generation of Li‐ion batteries, owing to its exceptional lithium storage capacity. The absence of cobalt in LRLO's composition provides an additional advantage, enabling cost‐effective production and thereby improving the feasibility of large‐scale manufacturing. Despite these promising attributes, LRLO has encountered challenges related to poor cycling performance and severe voltage decay, impeding its practical application. In addressing these challenges, a surface modification technique involving lithium borate (LBO) is employed through a dry coating method. The LBO‐coated LRLO exhibits a uniform surface layer with a thickness of 15 nm. Furthermore, the performance of LBO‐coated LRLO in a full cell is synergistically enhanced when combined with lithium bis(oxalato)borate (LiBOB) as an electrolyte additive. A discharge capacity retention of 82% is achieved after 400 cycles at room temperature. These substantial improvements are attributed to the continual reaction between boron species on the LRLO cathode surface and PF
了解硼化学作为无钴富锂层状氧化物的表面改性剂和电解质添加剂的作用
富锂层状氧化物(LRLO)因其卓越的锂储存能力而成为下一代锂离子电池极具潜力的正极材料。LRLO 成分中不含钴的另一个优势是,它的生产具有成本效益,从而提高了大规模生产的可行性。尽管 LRLO 具有这些良好的特性,但它也遇到了循环性能差和电压衰减严重等挑战,阻碍了它的实际应用。为了应对这些挑战,我们采用了一种涉及硼酸锂(LBO)的表面改性技术,通过干涂层的方法进行改性。硼酸锂涂层的 LRLO 显示出厚度为 15 纳米的均匀表面层。此外,当硼酸双(草酸)锂(LiBOB)作为电解质添加剂与硼酸双(草酸)锂结合使用时,硼酸双(草酸)锂涂层 LRLO 在全电池中的性能得到了协同增强。在室温下循环 400 次后,放电容量保持率达到 82%。这些重大改进归功于 LRLO 阴极表面的硼元素与电解液中的 PF6- 阴离子之间的持续反应。在高压充电过程中,这种反应会生成 BF4-,并抑制 HF 酸的形成,从而证明了 LRLO 在实际应用中的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Energy Materials
CHEMISTRY, PHYSICAL-ENERGY & FUELS
CiteScore
41.90
自引率
4.00%
发文量
889
审稿时长
1.4 months
期刊介绍:
Established in 2011, Advanced Energy Materials is an international, interdisciplinary, English-language journal that focuses on materials used in energy harvesting, conversion, and storage. It is regarded as a top-quality journal alongside Advanced Materials, Advanced Functional Materials, and Small.
With a 2022 Impact Factor of 27.8, Advanced Energy Materials is considered a prime source for the best energy-related research. The journal covers a wide range of topics in energy-related research, including organic and inorganic photovoltaics, batteries and supercapacitors, fuel cells, hydrogen generation and storage, thermoelectrics, water splitting and photocatalysis, solar fuels and thermosolar power, magnetocalorics, and piezoelectronics.
The readership of Advanced Energy Materials includes materials scientists, chemists, physicists, and engineers in both academia and industry. The journal is indexed in various databases and collections, such as Advanced Technologies & Aerospace Database, FIZ Karlsruhe, INSPEC (IET), Science Citation Index Expanded, Technology Collection, and Web of Science, among others.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


