Efficacy of once-nightly sodium oxybate (FT218) on daytime symptoms in individuals with narcolepsy with or without concomitant alerting agent use: A post hoc analysis from the phase 3 REST-ON trial
IF 3.8
2区 医学
Q1 CLINICAL NEUROLOGY
引用次数: 0
Abstract
Objective/Background
Extended-release, once-nightly sodium oxybate (ON-SXB) significantly improved narcolepsy symptoms in participants in the phase 3, randomized, double-blind, placebo-controlled REST-ON trial. This post hoc analysis of REST-ON data evaluated ON-SXB efficacy in participants with or without concomitant alerting agent use.
Patients/methods
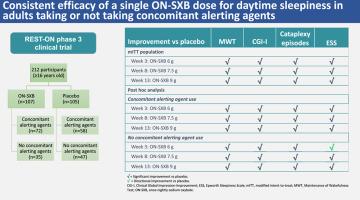
Participants with narcolepsy aged >16 years were randomized 1:1 to ON-SXB (week 1: 4.5 g, weeks 2–3: 6 g, weeks 4–8: 7.5 g, weeks 9–13: 9 g) or placebo. Primary endpoints in this post hoc analysis included change from baseline in mean sleep latency on the Maintenance of Wakefulness Test (MWT), Clinical Global Impression-Improvement (CGI-I) rating, and number of weekly cataplexy episodes. The secondary endpoints were change from baseline in the Epworth Sleepiness Scale (ESS) score and in objective and subjective disrupted nighttime sleep parameters. Post hoc analyses assessed participants with and without alerting agent use across 6-, 7.5-, and 9-g doses.
Results
In the modified intent-to-treat population, 119 (63 %) were (ON-SXB, n = 66; placebo, n = 53) and 71 (37 %) were not (ON-SXB, n = 31; placebo, n = 40) taking alerting agents. Regardless of alerting agent use, treatment with ON-SXB resulted in significant improvements vs placebo (all doses, P < 0.05) for MWT, CGI-I, and number of weekly cataplexy episodes. Significant improvements in ESS (all doses, P < 0.05) with ON-SXB vs placebo were observed in the alerting agent use cohort. Directional improvements in ESS were reported with all doses in the no alerting agent use group.
Conclusions
Regardless of concomitant alerting agent use, ON-SXB improved daytime and nighttime narcolepsy symptoms vs placebo.
无论是否同时使用警戒剂,每晚一次的羟苯甲酸钠(FT218)对嗜睡症患者白天症状的疗效:REST-ON 3 期试验的事后分析
目的/背景在第 3 期随机、双盲、安慰剂对照 REST-ON 试验中,每晚一次的羟苯甲酸钠缓释片(ON-SXB)能显著改善参与者的嗜睡症症状。这项REST-ON试验数据的事后分析评估了ON-SXB对同时使用或不使用警戒剂的参与者的疗效。患者/方法年龄为16岁的嗜睡症患者按1:1的比例随机接受ON-SXB(第1周:4.5克,第2-3周:6克,第4-8周:7.5克,第9-13周:9克)或安慰剂治疗。这项事后分析的主要终点包括维持清醒测试(MWT)的平均睡眠潜伏期、临床总体印象改善(CGI-I)评分和每周惊厥发作次数与基线相比的变化。次要终点是埃普沃思嗜睡量表(ESS)评分以及客观和主观夜间睡眠紊乱参数与基线相比的变化。结果在修改后的意向治疗人群中,119 人(63%)服用(ON-SXB,n = 66;安慰剂,n = 53),71 人(37%)未服用(ON-SXB,n = 31;安慰剂,n = 40)警示剂。无论是否使用警戒剂,使用 ON-SXB 与安慰剂相比(所有剂量,P < 0.05),MWT、CGI-I 和每周惊厥发作次数均有显著改善。在使用警戒剂的队列中,ON-SXB与安慰剂相比,ESS有明显改善(所有剂量,P< 0.05)。结论无论是否同时使用警戒剂,ON-SXB 与安慰剂相比都能改善白天和夜间的嗜睡症状。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Sleep medicine
医学-临床神经学
CiteScore
8.40
自引率
6.20%
发文量
1060
审稿时长
49 days
期刊介绍:
Sleep Medicine aims to be a journal no one involved in clinical sleep medicine can do without.
A journal primarily focussing on the human aspects of sleep, integrating the various disciplines that are involved in sleep medicine: neurology, clinical neurophysiology, internal medicine (particularly pulmonology and cardiology), psychology, psychiatry, sleep technology, pediatrics, neurosurgery, otorhinolaryngology, and dentistry.
The journal publishes the following types of articles: Reviews (also intended as a way to bridge the gap between basic sleep research and clinical relevance); Original Research Articles; Full-length articles; Brief communications; Controversies; Case reports; Letters to the Editor; Journal search and commentaries; Book reviews; Meeting announcements; Listing of relevant organisations plus web sites.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


