Alternative translation initiation produces synaptic organizer proteoforms with distinct localization and functions
IF 14.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
While many mRNAs contain more than one translation initiation site (TIS), the functions of most alternative TISs and their corresponding protein isoforms (proteoforms) remain undetermined. Here, we showed that alternative usage of CUG and AUG TISs in neuronal pentraxin receptor (NPR) mRNA produced two proteoforms, of which the ratio was regulated by RNA secondary structure and neuronal activity. Downstream AUG initiation truncated the N-terminal transmembrane domain and produced a secreted NPR proteoform sufficient in promoting synaptic clustering of AMPA-type glutamate receptors. Mutations that altered the ratio of NPR proteoforms reduced AMPA receptors in parvalbumin-positive interneurons and affected learning behaviors in mice. In addition to NPR, upstream AUU-initiated N-terminal extension of C1q-like synaptic organizers anchored these otherwise secreted factors to the membrane. Together, these results uncovered the plasticity of N-terminal signal sequences regulated by alternative TIS usage as a potentially widespread mechanism in diversifying protein localization and functions.
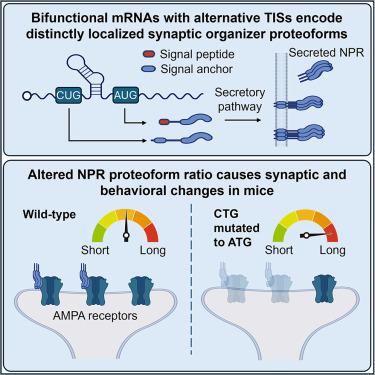
交替翻译起始产生的突触组织者蛋白形式具有不同的定位和功能
虽然许多 mRNA 都含有一个以上的翻译起始位点(TIS),但大多数替代性 TIS 的功能及其相应的蛋白质异构体(蛋白型)仍未确定。在这里,我们发现神经元五肽受体(NPR)mRNA 中 CUG 和 AUG TIS 的交替使用产生了两种蛋白形式,其比例受 RNA 二级结构和神经元活性的调节。下游 AUG 起始位点截断了 N 端跨膜结构域,产生的分泌型 NPR 蛋白形式足以促进 AMPA 型谷氨酸受体的突触集聚。改变 NPR 蛋白形式比例的突变会减少副发光体阳性中间神经元中的 AMPA 受体,并影响小鼠的学习行为。除 NPR 外,上游 AUU 引发的 C1q 样突触组织者的 N 端延伸也将这些原本分泌的因子固定在膜上。总之,这些结果揭示了受TIS替代用法调控的N端信号序列的可塑性,它可能是蛋白质定位和功能多样化的一种广泛机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Cell
生物-生化与分子生物学
CiteScore
26.00
自引率
3.80%
发文量
389
审稿时长
1 months
期刊介绍:
Molecular Cell is a companion to Cell, the leading journal of biology and the highest-impact journal in the world. Launched in December 1997 and published monthly. Molecular Cell is dedicated to publishing cutting-edge research in molecular biology, focusing on fundamental cellular processes. The journal encompasses a wide range of topics, including DNA replication, recombination, and repair; Chromatin biology and genome organization; Transcription; RNA processing and decay; Non-coding RNA function; Translation; Protein folding, modification, and quality control; Signal transduction pathways; Cell cycle and checkpoints; Cell death; Autophagy; Metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


