Ytterbium Triflate-Catalyzed Intramolecular Arylative Ring Opening of Arylaminomethyl-Substituted Donor–Acceptor Cyclopropanes: Access to Tetrahydroquinolines
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The treatment of arylaminomethyl-substituted donor–acceptor cyclopropanes with a catalytic amount of Yb(OTf)3 provides expedient access to tetrahydroquinoline derivatives. The transformation proceeds through an intramolecular SN2-type attack of the aminomethyl-containing aryl ring on the cyclopropane ring, leading to the formation of the products as single diastereomers.
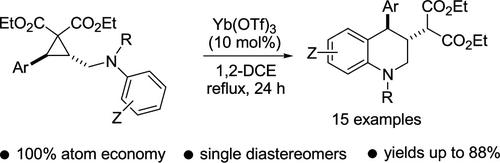
三氟化硼镱催化的芳基氨基甲基取代的供体-受体环丙烷分子内芳基化开环:获得四氢喹啉
用一定量的 Yb(OTf)3 催化剂处理芳基氨甲基取代的供体-受体环丙烷,可以快速获得四氢喹啉衍生物。这种转化是通过含氨甲基的芳基环对环丙烷环的分子内 SN2-型攻击进行的,从而形成单一非对映异构体的产物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


