Unraveling Lewis acid-base sites in defected MOFs for catalyzing dicyclopentadiene hydrogenation via a DFT study and descriptor exploration
Abstract
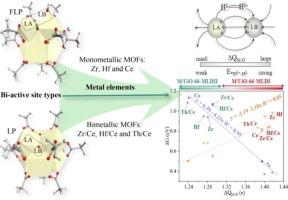
The 12-connected UiO-66 MOFs with great defect regulation ability have attracted significant attention due to the easily tunable electronic structure and surface electrostatic potential distribution of metal nodes. In this density functional theory (DFT) study, we systematically explored the effect of bi-active site types as the frustrated Lewis pair (FLP) and classical Lewis pair (LP) as well as metal elements of secondary building units (SBUs) on the catalytic hydrogenation of dicyclopentadiene (DCPD) to tetrahydrodicyclopentadiene (THDCPD). These design strategies effectively modulate the frontier molecular orbital energy level that determines the binding strength between MOFs and adsorbed species *Hδ+-Hδ- and charges transfer before and after H2 chemisorption. We also found that the heterolytic H2 adsorption energy and charges transfer linearly correlate with the energy barrier. In order to unravel the clear-cut designing strategies and further simplify computational complexity, we firstly proposed the ADCH charges difference between the Lewis acid and the Lewis base centers (ΔQM-O) as an intrinsic descriptor for catalytic activity of DCPD into THDCPD, which can effectively describe the reactivity and electrostatic effects of oppositely charged bi-active sites in the system. Furthermore, the defective UiO-66(Ce) MOF with moderate ΔQM-O effectively balance the contradiction between the barriers of two primitive reaction: the heterolytically H2 dissociation to *Hδ+-*Hδ- and the desorption of the active hydrogen species to hydrogenate 8,9-dihydrodicyclopentadiene (8,9-DHDCPD), resulting in the optimal catalytic performance. This work provides a deep understanding of the catalytic mechanism of MOFs with multiple active sites and might open up avenues for the rational design of novel hydrogenation catalysts.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


