Phase behavior of the carbon dioxide/toluene/poly(ethylene glycol) ternary system
Abstract
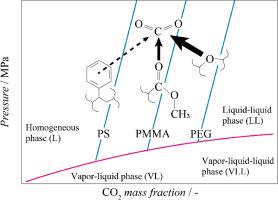
The phase behavior of a carbon dioxide (CO2)/toluene (Tol)/poly(ethylene glycol) (PEG) ternary system was investigated in this study to consider the effect of the polymer species on the phase diagram. Measurements were performed using a synthetic method combined with laser displacement and turbidity measurements. Bubble points (vapor–liquid phase separation) were determined from changes in the piston displacement and cloud points (liquid–liquid (LL) phase separation) were determined from changes in the turbidity. The phase boundaries of the CO2 mass fractions ranging from 0.113 to 0.496 were measured by varying the Tol/PEG mass ratio. The homogeneous phase area decreased when the mass ratio of PEG to Tol increased and/or the temperature decreased. These changes in the LL phase-separation behavior were explained by referring to the free volume fraction and solubility parameter estimated using the Sanchez–Lacombe equation of state. The free volume integral fraction could explain the phase diagram, but not the solubility parameter; the effect of polymer species on the Px phase diagram of the ternary system was explained by considering specific interactions between dissimilar components. These results could promote a comprehensive understanding of the phase diagram of CO2/organic solvent/polymer systems and aid the prediction of phase behavior.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


