Visible light-mediated radical addition cascade cyclization of aryl isocyanides with tricarbonyls: rapid access to substituted phenanthridines and isoquinolines.
IF 2.9
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A visible light-mediated synthesis of substituted phenanthridines and isoquinolines from ortho-substituted aryl isocyanides and tricarbonyl compounds is unveiled via the radical addition cascade cyclization (RACC) strategy. This acid/base-free method involves an oxidant (a persulphate salt) and a Ru-photocatalyst. This protocol avoids the use of halogenated compounds as pre-functionalized carbonyl precursors. The products can be easily post-modified to other important small molecules. The functional group tolerance of the reaction and the yields of the products are good without any scalability issues. The mechanistic investigation suggested the presence of a radical pathway during the reaction.
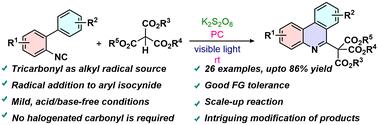
可见光介导的芳基异氰酸酯与三羰基的自由基加成级联环化反应:快速获得取代的菲啶类和异喹啉类化合物。
通过自由基加成级联环化(RACC)策略,揭示了一种以正交取代的芳基异氰酸酯和三羰基化合物为原料,在可见光介导下合成取代的菲啶类和异喹啉类化合物的方法。这种不含酸/碱的方法涉及一种氧化剂(过硫酸盐)和一种 Ru 光催化剂。该方案避免了使用卤代化合物作为预官能化的羰基前体。产物可以很容易地后修饰成其他重要的小分子。反应的官能团耐受性和产物的产量都很好,没有任何可扩展性问题。机理研究表明,反应过程中存在自由基途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
The international home of synthetic, physical and biomolecular organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


