Au(III)/TPPMS-catalyzed Friedel-Crafts benzylation of deactivated N-alkylanilines in water.
IF 2.9
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The Friedel-Crafts reaction of electronically deactivated anilines including those with strong electron-withdrawing NO2, CN or CO2H groups is challenging due to the reduced electron density of the aromatic ring. Here, we demonstrate the Au(III)/TPPMS-catalyzed Friedel-Crafts benzylation of deactivated anilines in water. This reaction exhibits operational simplicity and a broad substrate scope with high regioselectivity, enabling rapid access to 2-benzylanilines.
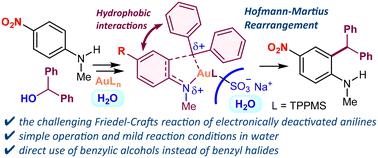
Au(III)/TPPMS 催化水中失活 N-烷基苯胺的 Friedel-Crafts 苄基化反应。
由于芳香环的电子密度降低,对电子失活苯胺(包括具有强吸电子 NO2、CN 或 CO2H 基团的苯胺)进行 Friedel-Crafts 反应具有挑战性。在此,我们展示了 Au(III)/TPPMS 催化的失活苯胺在水中的 Friedel-Crafts 苄基化反应。该反应操作简单,底物范围广,具有很高的区域选择性,可快速获得 2-苄基苯胺。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
The international home of synthetic, physical and biomolecular organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


