p-TSA catalyzed 6-endo-trig/dig cyclization of 5-aminopyrazoles and 3°/2°-propargylic alcohols: access to pyrazolo[1,5-a]dihydropyrimidines and pyrazolo[3,4-b]pyridines.
IF 2.9
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A facile, straightforward synthesis of fused pyrazolo[1,5-a]dihydropyrimidines and pyrazolo[3,4-b]pyridines is accomplished by using 5-aminopyrazoles, 3°/2°-propargylic alcohols and ynones in the presence of p-TSA. The reaction proceeds through allenylation (N-alkylation)/propargylation (C-alkylation) of 5-aminopyrazoles, followed by intramolecular 6-endo-trig/dig cyclization leading to the title products with the formation of new C-N and C-C bonds. Operationally simple reaction conditions, inexpensive reagents, better yields, and gram-scale synthesis are the advantages of this protocol.
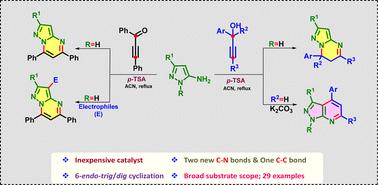
p-TSA 催化 5-氨基吡唑和 3°/2°-丙炔醇的 6-endo-trig/dig 环化反应:获得吡唑并[1,5-a]二氢嘧啶和吡唑并[3,4-b]吡啶。
在对-TSA 的存在下,利用 5-氨基吡唑、3°/2°-丙炔醇和炔酮,简便、直接地合成了融合的吡唑并[1,5-a]二氢嘧啶和吡唑并[3,4-b]吡啶。该反应通过 5-aminopyrazoles 的异戊烯化(N-烷基化)/丙炔化(C-烷基化)进行,然后进行分子内 6-endo-trig/dig 环化,生成新的 C-N 和 C-C 键,最终得到标题产物。该方法具有反应条件简单、试剂价格低廉、产率较高和克级合成等优点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
The international home of synthetic, physical and biomolecular organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


