Asymmetric Remote Aldol Cyclization Reaction to Synthesize Trifluoromethylated Heterospirocyclic Frameworks.
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
The Journal of Organic Chemistry
Pub Date : 2024-09-20
Epub Date: 2024-09-05
DOI:10.1021/acs.joc.4c01839
引用次数: 0
Abstract
The highly enantioselective organocatalytic synthesis of dihydropyran spirocyclic compounds bearing di- and trifluoromethyl groups by aldol cyclization reaction via trienamine using cyclic 2,5-dienones and different di- and trifluoromethylketones is described. Using a bifunctional aminothiourea catalyst, trifluoromethyl-functionalized dihydropyran spirocyclic products were obtained with good yields and enantioselectivities. Subsequent transformation with H2 and Pd/C has allowed the synthesis of the tetrahydropyran structure with three stereocenters. The plausible reaction mechanism was investigated by computational methods.
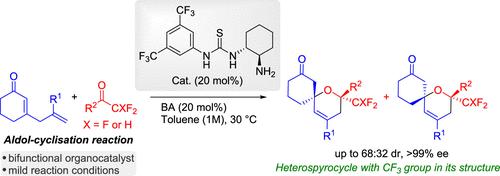
不对称远程醛醇环化反应合成三氟甲基异螺环框架。
本研究介绍了利用环状 2,5- 二烯酮和不同的二氟和三氟甲基酮,通过三烯胺进行醛醇环化反应,高度对映选择性地有机合成带有二氟和三氟甲基的二氢吡喃螺环化合物。通过使用双官能氨基硫脲催化剂,获得了三氟甲基官能化的二氢吡喃螺环产物,并具有良好的产率和对映选择性。随后用 H2 和 Pd/C 转化,合成了具有三个立体中心的四氢吡喃结构。通过计算方法研究了合理的反应机理。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


