Elastin-like polypeptide coacervates as reversibly triggerable compartments for synthetic cells
IF 5.9
2区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
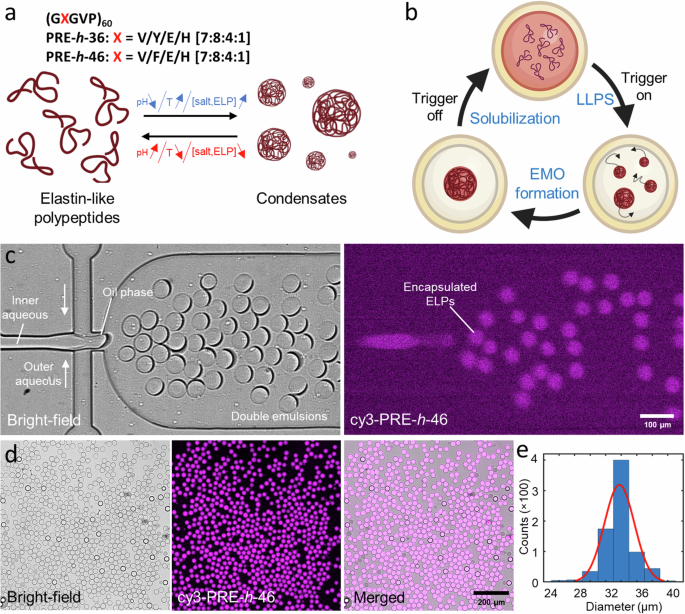
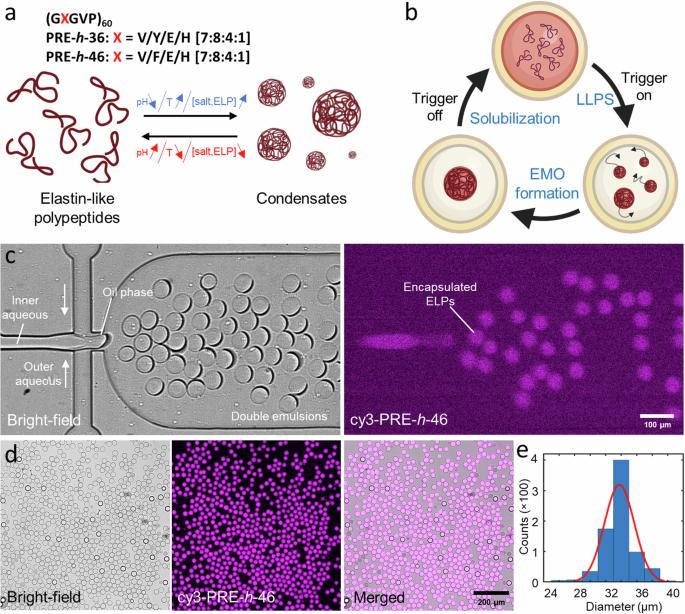
Compartmentalization is a vital aspect of living cells to orchestrate intracellular processes. In a similar vein, constructing dynamic and responsive sub-compartments is key to synthetic cell engineering. In recent years, liquid-liquid phase separation via coacervation has offered an innovative avenue for creating membraneless organelles (MOs) within artificial cells. Here, we present a lab-on-a-chip system to reversibly trigger peptide-based coacervates within cell-mimicking confinements. We use double emulsion droplets (DEs) as our synthetic cell containers while pH-responsive elastin-like polypeptides (ELPs) act as the coacervate system. We first present a high-throughput microfluidic DE production enabling efficient encapsulation of the ELPs. The DEs are then harvested to perform multiple MO formation-dissolution cycles using pH as well as temperature variation. For controlled long-term visualization and modulation of the external environment, we developed an integrated microfluidic device for trapping and environmental stimulation of DEs, with negligible mechanical force, and demonstrated a proof-of-principle osmolyte-based triggering to induce multiple MO formation-dissolution cycles. In conclusion, our work showcases the use of DEs and ELPs in designing membraneless reversible compartmentalization within synthetic cells via physicochemical triggers. Additionally, presented on-chip platform can be applied over a wide range of phase separation and vesicle systems for applications in synthetic cells and beyond. Compartmentalization within living cells is vital to orchestrate intracellular processes, but effective compartmentalization and organization within synthetic cells remains a key challenge. Here, the authors report a lab-on-a-chip system to reversibly trigger the formation of peptide-based coacervates as membraneless organelles via pH/temperature/osmolyte variations within cell-mimicking confinements.
弹性蛋白样多肽凝聚物作为合成细胞的可逆触发隔室
区隔化是活细胞协调细胞内过程的一个重要方面。同样,构建动态和反应灵敏的亚细胞器也是合成细胞工程的关键。近年来,通过共凝的液-液相分离为在人造细胞内创建无膜细胞器(MO)提供了一条创新途径。在这里,我们展示了一种片上实验室系统,用于在模拟细胞的限制条件下可逆地触发基于肽的共凝物。我们使用双乳液液滴(DEs)作为合成细胞容器,而 pH 响应型弹性蛋白样多肽(ELPs)则作为凝聚剂系统。我们首先介绍了一种高通量微流控双乳液滴生产技术,它能有效地封装 ELPs。然后收获 DE,利用 pH 值和温度变化执行多个 MO 形成-溶解循环。为了实现可控的长期可视化和外部环境调控,我们开发了一种用于捕获和环境刺激 DEs 的集成微流控装置,其机械力可以忽略不计,并展示了一种基于渗透剂的触发原理验证,可诱导多个 MO 形成-溶解循环。总之,我们的工作展示了如何利用 DEs 和 ELPs 通过物理化学触发器在合成细胞内设计无膜可逆区隔。此外,所展示的片上平台可广泛应用于合成细胞内外的相分离和囊泡系统。活细胞内的区隔对于协调细胞内过程至关重要,但合成细胞内有效的区隔和组织仍然是一个关键挑战。在此,作者报告了一种片上实验室系统,该系统在模拟细胞的限制条件下,通过 pH 值/温度/溶质的变化,可逆地触发基于肽的凝聚态形成无膜细胞器。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Communications Chemistry
Chemistry-General Chemistry
CiteScore
7.70
自引率
1.70%
发文量
146
审稿时长
13 weeks
期刊介绍:
Communications Chemistry is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the chemical sciences. Research papers published by the journal represent significant advances bringing new chemical insight to a specialized area of research. We also aim to provide a community forum for issues of importance to all chemists, regardless of sub-discipline.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


