Organic Bases as the Organic Electron Donors (OED) Promoted Reductive Coupling of Diarylhalomethanes: Halogens Controlled Construction of Tetraarylethylenes and Tetraarylethanes.
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
The Journal of Organic Chemistry
Pub Date : 2024-09-20
Epub Date: 2024-09-02
DOI:10.1021/acs.joc.4c01219
引用次数: 0
Abstract
Using the organic base as the organic electron donors, the reductive coupling of diaryhalomethanes was smoothly achieved under transition-metal-free reaction conditions, giving a series of synthetically important tetraarylethylenes and tetraarylethanes in high yields. The mechanistic study revealed that the organic bases acting as the electron donor initiated the generation of a radical intermediate, realizing the construction of tetraarylethylene and tetraarylethane skeletons.
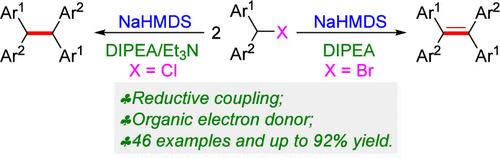
作为有机电子供体(OED)的有机碱促进二芳基卤代甲烷的还原偶联:卤素控制的四芳基乙烯和四芳基乙烷的构建。
以有机碱为有机电子供体,在无过渡金属反应条件下顺利实现了二芳基卤代甲烷的还原偶联,高产率地得到了一系列具有重要合成意义的四芳基乙烯和四芳基乙烷。机理研究表明,作为电子供体的有机碱启动了自由基中间体的生成,实现了四芳基乙烯和四芳基乙烷骨架的构建。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


