Using proteomics to identify the mechanisms underlying the benefits of statins on ischemic heart disease
引用次数: 0
Abstract
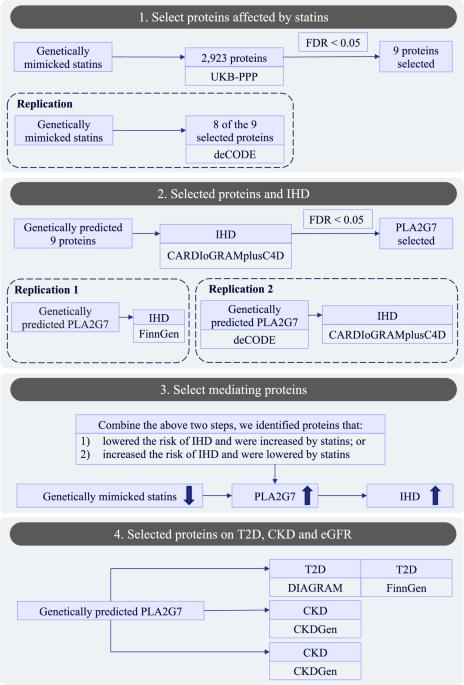
Ischemic heart disease (IHD) is the single leading cause of mortality globally. Statins are the mainstay for IHD treatment. However, the specific mechanisms underlying statins’ benefits on IHD have not been clarified. To examine the mechanisms through proteins, we used two-step Mendelian randomization (MR) approach. First, we examined the associations of genetically mimicked statins with 2923 proteins using genome-wide association of proteins from the UK Biobank Pharma Proteomics Project (UKB-PPP) to identify the proteins affected by statins, and replicated the findings using deCODE. Then we examined the associations of selected proteins with IHD risk using CARDIoGRAMplusC4D using MR, and replicated using FinnGen, and using another set of genetic instruments from deCODE. We selected proteins decreased or increased IHD risk and meanwhile increased or lowered by statins. We further examined the role of the selected protein(s) on common IHD comorbidities, including diabetes, chronic kidney disease (CKD), and kidney function (measured by estimated glomerular filtration rate (eGFR)). Nine proteins were affected by statins, including four proteins (PLA2G7, FGFBP1, ANGPTL1, and PTPRZ1) lowered by statins, and five proteins (EFNA4, COL6A3, ASGR1, PRSS8 and PCOLCE) increased by statins. Among these, PLA2G7 was related to higher risk of IHD after controlling for multiple testing. The associations were robust to different analytic methods and replication using another set of genetic instrument from deCODE, and using another GWAS of IHD from FinnGen. Genetically predicted PLA2G7 had null association with diabetes, CKD, and eGFR. We identified 9 proteins affected by statins, including 7 novel proteins which were not reported previously. PLA2G7 is on the pathway underlying statins’ benefits on IHD. The clarification of statins’ mechanisms had close relevance to precision medicine, and provided insights to the development of new treatment strategies.
利用蛋白质组学确定他汀类药物治疗缺血性心脏病的机制
缺血性心脏病(IHD)是导致全球死亡的唯一主要原因。他汀类药物是治疗缺血性心脏病的主要药物。然而,他汀类药物治疗 IHD 的具体机制尚未明确。为了通过蛋白质研究其机制,我们采用了两步孟德尔随机法(MR)。首先,我们利用英国生物库医药蛋白质组学项目(UKB-PPP)的蛋白质全基因组关联研究了基因模拟他汀与 2923 种蛋白质的关联,以确定受他汀影响的蛋白质,并利用 deCODE 复制了研究结果。然后,我们利用MR使用CARDIoGRAMplusC4D检验了所选蛋白质与IHD风险的关联,并利用FinnGen和deCODE的另一组遗传工具进行了复制。我们选择的蛋白质会降低或增加 IHD 风险,同时他汀类药物会增加或降低 IHD 风险。我们进一步研究了所选蛋白质对常见 IHD 并发症的作用,包括糖尿病、慢性肾病 (CKD) 和肾功能(通过估算肾小球滤过率 (eGFR) 测量)。他汀类药物影响了九种蛋白质,其中四种蛋白质(PLA2G7、FGFBP1、ANGPTL1和PTPRZ1)受他汀类药物影响而降低,五种蛋白质(EFNA4、COL6A3、ASGR1、PRSS8和PCOLCE)受他汀类药物影响而升高。其中,PLA2G7在控制多重检测后与较高的IHD风险有关。基因预测的 PLA2G7 与糖尿病、慢性肾脏病和 eGFR 的关系为零。我们发现了9种受他汀类药物影响的蛋白质,包括7种以前未报道过的新型蛋白质。PLA2G7是他汀类药物治疗高血压的基本途径。阐明他汀类药物的作用机制与精准医疗密切相关,并为开发新的治疗策略提供了见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


