Discovery of 6,7-Dihydropyrazolo[1,5-a]pyrazin-4(5H)-one Derivatives as mGluR2 Negative Allosteric Modulators with In Vivo Activity in a Rodent’s Model of Cognition
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
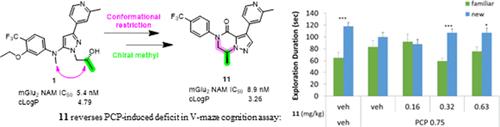
Allosteric modulators of the metabotropic group II receptors, mGluR2 and mGluR3, have been widely explored due to their ability to modulate cognitive and neurological functions in mood disorders, although none have been approved yet. In our search for new and selective mGluR2 negative allosteric modulators (NAMs), series of 6,7-dihydropyrazolo[1,5-a]pyrazin-4(5H)-one derivatives were identified from our published series of 1,3,5-trisubstituted pyrazoles. SAR evolution of the initial hit resulted in 100-fold improvement in the mGluR2 NAM potency and subsequent selection of compound 11 based on its overall profile, including selectivity and ADMET properties. Further pharmacokinetic-pharmacodynamic (PK–PD) relationship built showed that compound 11 occupied the mGluR2 receptor in a dose-dependent manner. Additionally, the compound revealed in vivo activity in V-maze as a model of cognition from a dose of 0.32 mg/kg. Compound 11 was selected to be evaluated further.
在啮齿动物认知模型中发现 6,7-二氢吡唑并[1,5-a]吡嗪-4(5H)-酮衍生物作为具有体内活性的 mGluR2 负异构调节剂
代谢组 II 受体 mGluR2 和 mGluR3 的异位调节剂因其能够调节情绪障碍患者的认知和神经功能而受到广泛关注,但目前还没有一种异位调节剂获得批准。在寻找新的选择性 mGluR2 负性异位调节剂(NAMs)的过程中,我们从已发表的 1,3,5-三取代吡唑系列中发现了一系列 6,7-二氢吡唑并[1,5-a]吡嗪-4(5H)-酮衍生物。对最初发现的化合物进行 SAR 演化后,mGluR2 NAM 的效力提高了 100 倍,随后根据其整体特征(包括选择性和 ADMET 特性)选择了化合物 11。进一步建立的药代动力学-药效学(PK-PD)关系显示,化合物 11 以剂量依赖的方式占据了 mGluR2 受体。此外,该化合物在作为认知模型的 V 型迷宫中显示出体内活性,剂量为 0.32 毫克/千克。化合物 11 被选中进行进一步评估。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


