Parallel loss of sexual reproduction in field populations of a brown alga sheds light on the mechanisms underlying the emergence of asexuality
IF 13.9
1区 生物学
Q1 ECOLOGY
引用次数: 0
Abstract
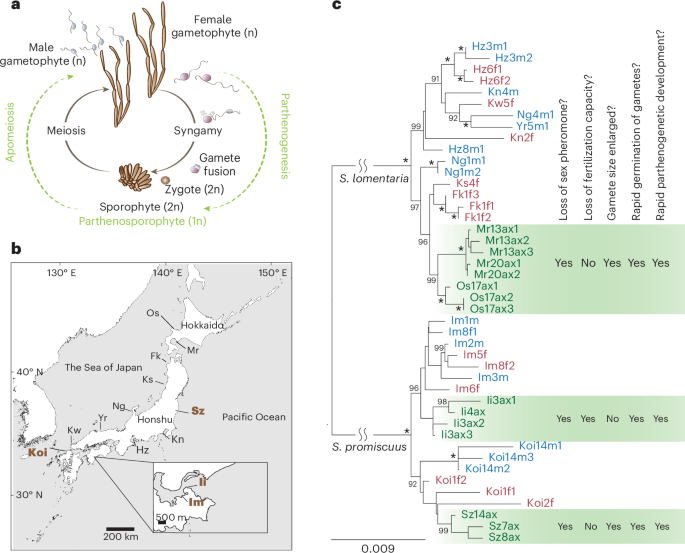
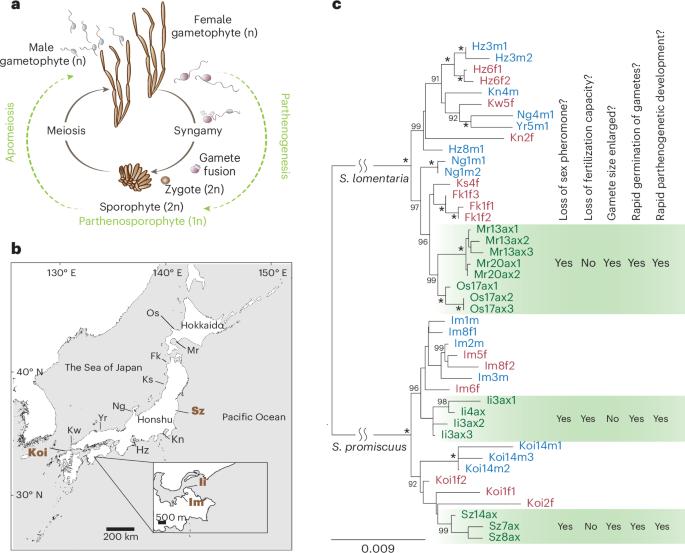
Sexual reproduction is widespread, but asexual lineages have repeatedly arisen from sexual ancestors across a wide range of eukaryotic taxa. The molecular changes underpinning the switch to asexuality remain elusive, particularly in organisms with haploid sexual systems. Here we explore independent events of loss of sex in the brown alga Scytosiphon, examine the proximate and evolutionary mechanisms involved, and test the importance of sexual conflict on gene expression changes following loss of sex. We find that asexual females (‘Amazons’) lose ability to produce sex pheromone and, consequently, are incapable of attracting males, whereas they gain rapid parthenogenic development from large, unfertilized eggs. These phenotypic changes are accompanied by convergent changes in gene expression. Decay of female functions, rather than relaxation of sexual antagonism, may be a dominant force at play during the emergence of asexuality in haploid sexual systems. Moreover, we show that haploid purifying selection plays a key role in limiting the accumulation of deleterious alleles in Amazons, and we identify an autosomal locus associated with the Amazon phenotype. The sex chromosome, together with this autosomal locus, may underlie the switch to obligate asexuality in the Amazon populations. Analysis of gamete transcriptomes of sexual and asexual individuals of the brown alga Scytosiphon shows decay of female functions but no signature of relaxation of sexual antagonism in the evolution of asexual reproduction in this haploid sexual system.
褐藻田间种群有性生殖的平行丧失揭示了无性生殖出现的内在机制
有性生殖非常普遍,但在众多真核生物类群中,无性系却一再从有性祖先中产生。特别是在具有单倍体有性系统的生物中,无性繁殖的分子变化仍然难以捉摸。在这里,我们探讨了褐藻 Scytosiphon 中丧失性别的独立事件,研究了其中涉及的近因和进化机制,并检验了性冲突对丧失性别后基因表达变化的重要性。我们发现,无性雌藻("Amazons")失去了产生性信息素的能力,因此无法吸引雄藻,而它们却能从大的未受精卵中获得快速孤雌生殖发育。这些表型变化伴随着基因表达的趋同变化。在单倍体有性系统中,雌性功能的衰退,而不是性对抗的放松,可能是无性出现过程中的主导力量。此外,我们还发现单倍体纯化选择在限制亚马逊雌性中有害等位基因的积累方面发挥了关键作用,并确定了一个与亚马逊表型相关的常染色体位点。性染色体和这个常染色体位点可能是亚马逊种群转为强制性无性繁殖的基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature ecology & evolution
Agricultural and Biological Sciences-Ecology, Evolution, Behavior and Systematics
CiteScore
22.20
自引率
2.40%
发文量
282
期刊介绍:
Nature Ecology & Evolution is interested in the full spectrum of ecological and evolutionary biology, encompassing approaches at the molecular, organismal, population, community and ecosystem levels, as well as relevant parts of the social sciences. Nature Ecology & Evolution provides a place where all researchers and policymakers interested in all aspects of life's diversity can come together to learn about the most accomplished and significant advances in the field and to discuss topical issues. An online-only monthly journal, our broad scope ensures that the research published reaches the widest possible audience of scientists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


