I2/CAN as an efficient reagent for the direct oxidation of tertiary Csp3-H to Csp3-OH: Synthesis of bis(N-methyl-7-azaindol-3-yl) (phenyl) methanol from 3,3′-(phenyl methylene)-bis(N-methyl-7-azaindole)
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
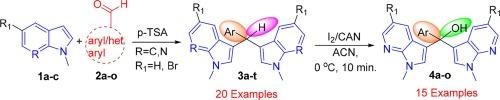
I2/CAN has emerged as a powerful and efficient oxidizing agent for direct tertiary CH oxidation of 3,3′-(phenyl methylene) bis(N-methyl-7-azaindole) under mild conditions to form bis(N-methyl-7-azaindol-3-yl) (phenyl)methanol in quantitative yield. Further, based on control experiments, a plausible mechanism and characterization of products including XRD are described. Synthetic transformation for bis(N-methyl-7-azaindol-3-yl) (phenyl) methanol is achieved by thionation.
I2/CAN 作为三级 Csp3-H 直接氧化为 Csp3-OH 的高效试剂:从 3,3′-(苯基亚甲基)-双(N-甲基-7-氮杂吲哚)合成双(N-甲基-7-氮杂吲哚-3-基)(苯基)甲醇
I/CAN 是一种强力高效的氧化剂,可在温和的条件下直接对 3,3′-(苯基亚甲基)双(-甲基-7-氮杂吲哚)进行三级 CH 氧化,生成双(-甲基-7-氮杂吲哚-3-基)(苯基)甲醇,产率定量。此外,在对照实验的基础上,还描述了一种合理的机理和包括 XRD 在内的产品特征。双(-甲基-7-氮杂吲哚-3-基)(苯基)甲醇的合成转化是通过亚硫酰化反应实现的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


