Angiopoietin-like 4 protects against endothelial dysfunction during bacterial sepsis
IF 20.5
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
Abstract
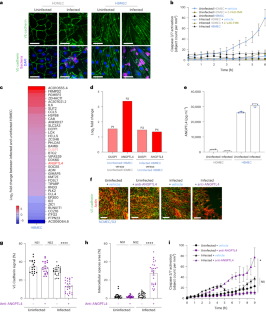
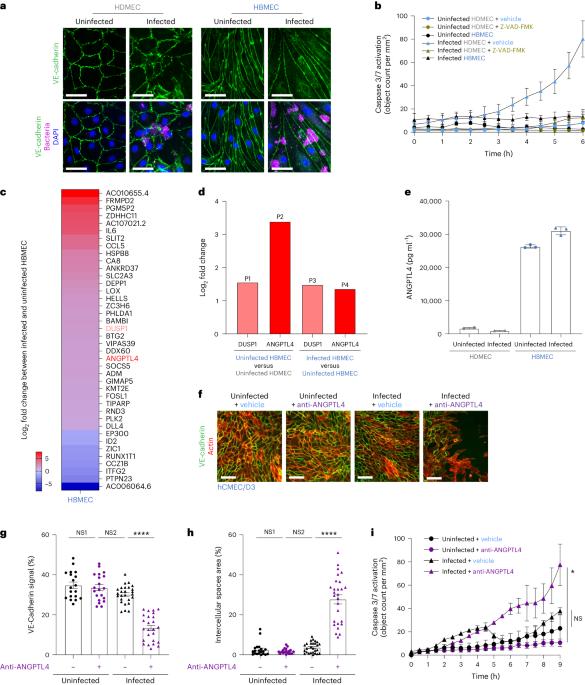
Loss of endothelial integrity and vascular leakage are central features of sepsis pathogenesis; however, no effective therapeutic mechanisms for preserving endothelial integrity are available. Here we show that, compared to dermal microvessels, brain microvessels resist infection by Neisseria meningitidis, a bacterial pathogen that causes sepsis and meningitis. By comparing the transcriptional responses to infection in dermal and brain endothelial cells, we identified angiopoietin-like 4 as a key factor produced by the brain endothelium that preserves blood–brain barrier integrity during bacterial sepsis. Conversely, angiopoietin-like 4 is produced at lower levels in the peripheral endothelium. Treatment with recombinant angiopoietin-like 4 reduced vascular leakage, organ failure and death in mouse models of lethal sepsis and N. meningitidis infection. Protection was conferred by a previously uncharacterized domain of angiopoietin-like 4, through binding to the heparan proteoglycan, syndecan-4. These findings reveal a potential strategy to prevent endothelial dysfunction and improve outcomes in patients with sepsis. Therapeutic administration of angiopoietin-like 4 prevents shock during Neisseria meningitidis infection or lipopolysaccharide-induced sepsis in mice.
血管生成素样 4 可防止细菌性败血症期间的内皮功能障碍
内皮完整性丧失和血管渗漏是败血症发病机制的核心特征;然而,目前还没有保护内皮完整性的有效治疗机制。在这里,我们发现与真皮微血管相比,脑微血管能抵抗脑膜炎奈瑟菌的感染,脑膜炎奈瑟菌是一种能导致败血症和脑膜炎的细菌病原体。通过比较皮肤内皮细胞和脑内皮细胞对感染的转录反应,我们发现血管生成素样 4 是脑内皮细胞产生的一种关键因子,它能在细菌性败血症期间保持血脑屏障的完整性。相反,外周内皮细胞产生的血管生成素样 4 水平较低。用重组类血管生成素 4 治疗致命性败血症和脑膜炎双球菌感染小鼠模型,可减少血管渗漏、器官衰竭和死亡。血管生成素样 4 的一个先前未表征的结构域通过与肝糖蛋白多糖 syndecan-4 结合而提供保护。这些发现揭示了一种预防内皮功能障碍和改善败血症患者预后的潜在策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


