In vitro resistance development gives insights into molecular resistance mechanisms against cefiderocol
IF 2.1
4区 医学
Q3 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
Abstract
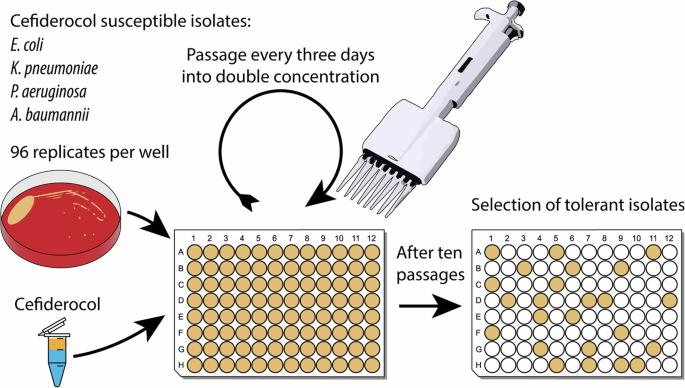
Cefiderocol, a novel siderophore cephalosporin, demonstrates promising in vitro activity against multidrug-resistant Gram-negative bacteria, including carbapenemase-producing strains. Nonetheless, only a few reports are available regarding the acquisition of resistance in clinical settings, primarily due to its recent usage. This study aimed to investigate cefiderocol resistance using an in vitro resistance development model to gain insights into the underlying molecular resistance mechanisms. Cefiderocol susceptible reference strains (Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa) and a clinical Acinetobacter baumannii complex isolate were exposed to increasing cefiderocol concentrations using a high-throughput resistance development model. Cefiderocol susceptibility testing was performed using broth microdilution. Whole-genome sequencing was employed to identify newly acquired resistance mutations. Our in vitro resistance development model led to several clones of strains exhibiting cefiderocol resistance, with MIC values 8-fold to 512-fold higher than initial levels. In total, we found 42 different mutations in 26 genes, of which 35 could be described for the first time. Putative loss-of-function mutations were detected in the envZ, tonB, and cirA genes in 13 out of 17 isolates, leading to a decrease in cefiderocol influx. Other potential resistance mechanisms included multidrug efflux pumps (baeS, czcS, nalC), antibiotic-inactivating enzymes (ampR, dacB), and target mutations in penicillin-binding-protein genes (mrcB). This study reveals new insights into underlying molecular resistance mechanisms against cefiderocol. While mutations leading to reduced influx via iron transporters was the most frequent resistance mechanism, we also detected several other novel resistance mutations causing cefiderocol resistance.
体外抗药性的发展让人们了解到头孢哌酮的分子抗药性机制。
Cefiderocol 是一种新型嗜苷头孢菌素,在体外对耐多药革兰氏阴性菌(包括产碳青霉烯酶的菌株)具有良好的活性。然而,关于头孢菌素在临床环境中产生耐药性的报道却寥寥无几,这主要是因为头孢菌素最近才开始使用。本研究旨在利用体外耐药性发展模型研究头孢羟氨苄的耐药性,以深入了解其潜在的分子耐药性机制。利用高通量耐药性发展模型,将头孢德醇易感参考菌株(大肠埃希菌、肺炎克雷伯菌、铜绿假单胞菌)和临床鲍曼不动杆菌复合分离株暴露于浓度不断增加的头孢德醇中。采用肉汤微稀释法进行头孢羟氨苄药敏试验。采用全基因组测序来鉴定新获得的耐药性突变。我们的体外耐药性发展模型产生了多个表现出头孢羟氨苄耐药性的菌株克隆,其 MIC 值是初始水平的 8 倍到 512 倍。我们总共在 26 个基因中发现了 42 种不同的突变,其中 35 种是首次发现。在 17 个分离株中的 13 个中检测到了 envZ、tonB 和 cirA 基因的假定功能缺失突变,从而导致头孢羟氨苄的流入量减少。其他潜在的抗药性机制包括多药外排泵(baeS、czcS、nalC)、抗生素失活酶(ampR、dacB)以及青霉素结合蛋白基因(mrcB)的靶突变。这项研究揭示了头孢菌素耐药性的分子机制。虽然导致铁转运体流入量减少的突变是最常见的耐药机制,但我们还发现了其他几种导致头孢克洛耐药的新型耐药突变。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Antibiotics
医学-免疫学
CiteScore
6.60
自引率
3.00%
发文量
87
审稿时长
1 months
期刊介绍:
The Journal of Antibiotics seeks to promote research on antibiotics and related types of biologically active substances and publishes Articles, Review Articles, Brief Communication, Correspondence and other specially commissioned reports. The Journal of Antibiotics accepts papers on biochemical, chemical, microbiological and pharmacological studies. However, studies regarding human therapy do not fall under the journal’s scope. Contributions regarding recently discovered antibiotics and biologically active microbial products are particularly encouraged. Topics of particular interest within the journal''s scope include, but are not limited to, those listed below:
Discovery of new antibiotics and related types of biologically active substances
Production, isolation, characterization, structural elucidation, chemical synthesis and derivatization, biological activities, mechanisms of action, and structure-activity relationships of antibiotics and related types of biologically active substances
Biosynthesis, bioconversion, taxonomy and genetic studies on producing microorganisms, as well as improvement of production of antibiotics and related types of biologically active substances
Novel physical, chemical, biochemical, microbiological or pharmacological methods for detection, assay, determination, structural elucidation and evaluation of antibiotics and related types of biologically active substances
Newly found properties, mechanisms of action and resistance-development of antibiotics and related types of biologically active substances.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


