Non-invasive mapping of brown adipose tissue activity with magnetic resonance imaging
IF 18.9
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
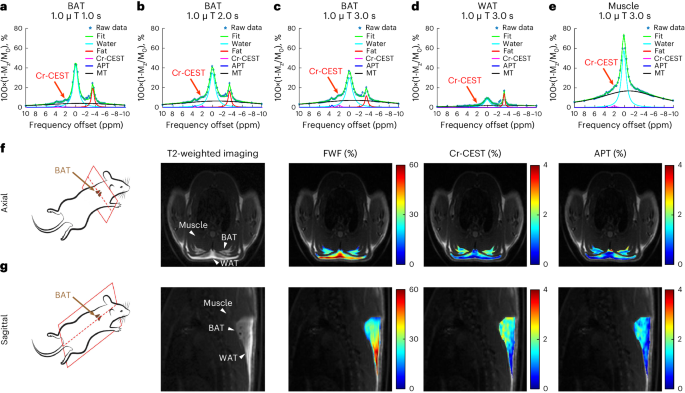
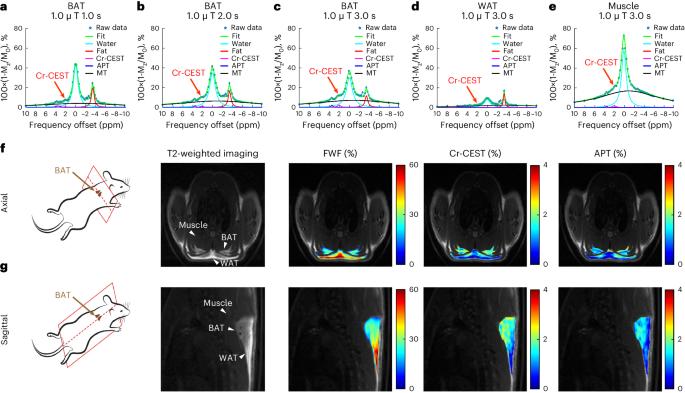
Thermogenic brown adipose tissue (BAT) has a positive impact on whole-body metabolism. However, in vivo mapping of BAT activity typically relies on techniques involving ionizing radiation, such as [18F]fluorodeoxyglucose ([18F]FDG) positron emission tomography (PET) and computed tomography (CT). Here we report a noninvasive metabolic magnetic resonance imaging (MRI) approach based on creatine chemical exchange saturation transfer (Cr-CEST) contrast to assess in vivo BAT activity in rodents and humans. In male rats, a single dose of the β3-adrenoceptor agonist (CL 316,243) or norepinephrine, as well as cold exposure, triggered a robust elevation of the Cr-CEST MRI signal, which was consistent with the [18F]FDG PET and CT data and 1H nuclear magnetic resonance measurements of creatine concentration in BAT. We further show that Cr-CEST MRI detects cold-stimulated BAT activation in humans (both males and females) using a 3T clinical scanner, with data-matching results from [18F]FDG PET and CT measurements. This study establishes Cr-CEST MRI as a promising noninvasive and radiation-free approach for in vivo mapping of BAT activity. Creatine chemical exchange saturation transfer magnetic resonance imaging is used successfully to measure brown adipose tissue activity in rats and humans, delivering data that are consistent with [18F]fluorodeoxyglucose PET and CT measurements.
利用磁共振成像对棕色脂肪组织的活动进行无创绘图。
产热性棕色脂肪组织(BAT)对全身新陈代谢有积极影响。然而,体内绘制棕色脂肪组织活性图通常依赖于涉及电离辐射的技术,如[18F]氟脱氧葡萄糖([18F]FDG)正电子发射断层扫描(PET)和计算机断层扫描(CT)。在此,我们报告了一种基于肌酸化学交换饱和转移(Cr-CEST)对比的无创代谢磁共振成像(MRI)方法,用于评估啮齿类动物和人类体内 BAT 的活性。在雄性大鼠体内,单剂量的 β3-肾上腺素受体激动剂(CL 316,243)或去甲肾上腺素以及寒冷暴露会引发 Cr-CEST MRI 信号的强烈升高,这与 BAT 中肌酸浓度的[18F]FDG PET 和 CT 数据以及 1H 核磁共振测量结果一致。我们进一步表明,使用 3T 临床扫描仪,Cr-CEST MRI 可检测到人体(男性和女性)冷刺激下的 BAT 激活,其数据与 [18F]FDG PET 和 CT 测量结果相匹配。这项研究证明,Cr-CEST MRI 是一种有望在体内绘制 BAT 活动图的无创、无辐射方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


