Tumor evolution metrics predict recurrence beyond 10 years in locally advanced prostate cancer
IF 23.5
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
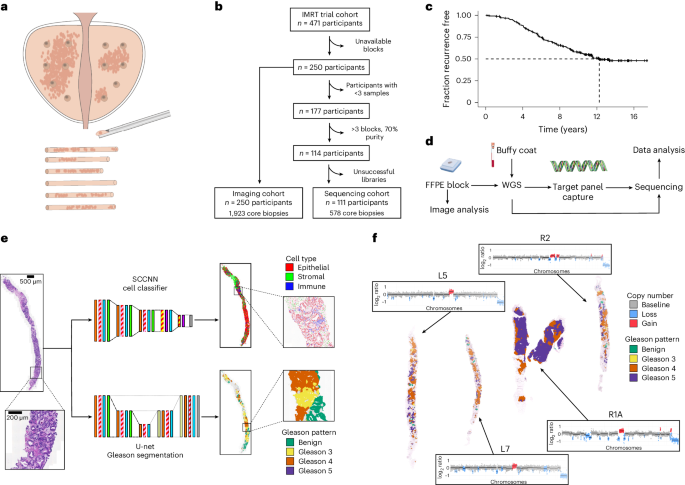
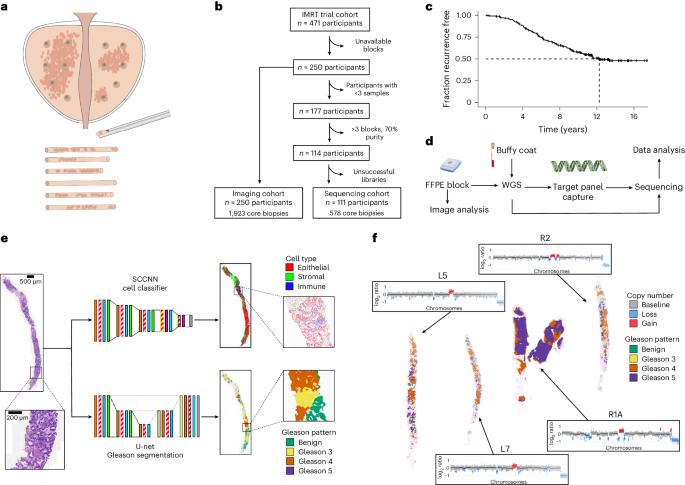
Cancer evolution lays the groundwork for predictive oncology. Testing evolutionary metrics requires quantitative measurements in controlled clinical trials. We mapped genomic intratumor heterogeneity in locally advanced prostate cancer using 642 samples from 114 individuals enrolled in clinical trials with a 12-year median follow-up. We concomitantly assessed morphological heterogeneity using deep learning in 1,923 histological sections from 250 individuals. Genetic and morphological (Gleason) diversity were independent predictors of recurrence (hazard ratio (HR) = 3.12 and 95% confidence interval (95% CI) = 1.34–7.3; HR = 2.24 and 95% CI = 1.28–3.92). Combined, they identified a group with half the median time to recurrence. Spatial segregation of clones was also an independent marker of recurrence (HR = 2.3 and 95% CI = 1.11–4.8). We identified copy number changes associated with Gleason grade and found that chromosome 6p loss correlated with reduced immune infiltration. Matched profiling of relapse, decades after diagnosis, confirmed that genomic instability is a driving force in prostate cancer progression. This study shows that combining genomics with artificial intelligence-aided histopathology leads to the identification of clinical biomarkers of evolution. Sottoriva and colleagues combine next-generation sequencing and AI-aided histopathology to assess tumor evolvability in patient samples with long-term follow-up and find that it can be a strong predictor of recurrence in high-risk prostate cancer.
肿瘤演变指标可预测局部晚期前列腺癌 10 年后的复发。
癌症演变为预测性肿瘤学奠定了基础。测试进化指标需要在对照临床试验中进行定量测量。我们利用临床试验中114人的642份样本绘制了局部晚期前列腺癌的肿瘤内基因组异质性图谱,中位随访期为12年。同时,我们还利用深度学习对来自 250 名患者的 1923 个组织学切片进行了形态学异质性评估。遗传和形态学(Gleason)多样性是复发的独立预测因素(危险比 (HR) = 3.12,95% 置信区间 (95% CI) = 1.34-7.3;HR = 2.24,95% CI = 1.28-3.92)。综合来看,他们发现了一个复发时间中位数为一半的群体。克隆的空间隔离也是复发的独立标志(HR = 2.3,95% CI = 1.11-4.8)。我们确定了与格里森分级相关的拷贝数变化,并发现染色体 6p 缺失与免疫浸润减少相关。对诊断后数十年的复发进行匹配分析,证实基因组不稳定性是前列腺癌进展的驱动力。这项研究表明,将基因组学与人工智能辅助组织病理学相结合,可以鉴定出进化的临床生物标志物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature cancer
Medicine-Oncology
CiteScore
31.10
自引率
1.80%
发文量
129
期刊介绍:
Cancer is a devastating disease responsible for millions of deaths worldwide. However, many of these deaths could be prevented with improved prevention and treatment strategies. To achieve this, it is crucial to focus on accurate diagnosis, effective treatment methods, and understanding the socioeconomic factors that influence cancer rates.
Nature Cancer aims to serve as a unique platform for sharing the latest advancements in cancer research across various scientific fields, encompassing life sciences, physical sciences, applied sciences, and social sciences. The journal is particularly interested in fundamental research that enhances our understanding of tumor development and progression, as well as research that translates this knowledge into clinical applications through innovative diagnostic and therapeutic approaches. Additionally, Nature Cancer welcomes clinical studies that inform cancer diagnosis, treatment, and prevention, along with contributions exploring the societal impact of cancer on a global scale.
In addition to publishing original research, Nature Cancer will feature Comments, Reviews, News & Views, Features, and Correspondence that hold significant value for the diverse field of cancer research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


