Tungsten-Catalyzed Regioselective Allylic Amination
IF 1.7
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A highly regioselective synthesis of allylic amines based on a tungsten-catalyzed allylic amination has been developed. This protocol, which is catalyzed by commercially available W(CO)3(MeCN)3 and 4,4′-di-tert-butyl-2,2′-bipyridine, permits the formation of synthetically useful branched allylic N-aryl- and N-alkylamines in moderate to good yields with a >20:1 branched/linear ratio under mild conditions. The noble-metal-free catalytic system complements conventional allylic aminations catalyzed by an Ir or Rh complex.
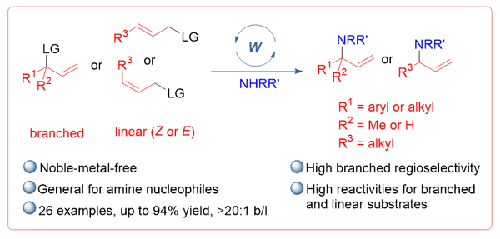
钨催化的区域选择性烯丙基胺反应
在钨催化烯丙基胺化的基础上,开发出了一种高区域选择性的烯丙基胺合成方法。该方案由市场上可买到的 W(CO)3(MeCN)3 和 4,4′-二叔丁基-2,2′-联吡啶催化,可在温和的条件下以中等至良好的产率形成合成上有用的支链烯丙基 N-芳基和 N-烷基胺,支链/线性比例大于 20:1。这种不含贵金属的催化系统补充了由 Ir 或 Rh 复合物催化的传统烯丙基胺化反应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Synlett
化学-有机化学
CiteScore
3.40
自引率
5.00%
发文量
369
审稿时长
1 months
期刊介绍:
SYNLETT is an international journal reporting research results and current trends in chemical synthesis in short personalized reviews and preliminary communications. It covers all fields of scientific endeavor that involve organic synthesis, including catalysis, organometallic, medicinal, biological, and photochemistry, but also related disciplines and offers the possibility to publish scientific primary data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


