1,6-Conjugate addition of in situ generated aryldiazenes to p-quinone methides†
IF 2.9
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Herein we report a transition-metal free, base-mediated 1,6-conjugate addition of aryldiazenes to para-quinone methides (p-QMs). Arylhydrazines were used for the in situ generation of aryldiazenes using a base-mediated protocol in the presence of air as the oxidant. The 1,6-conjugate addition of aryldiazenes to para-quinone methides via a radical mechanism is followed by an oxidative rearrangement to furnish the desired product, arylhydrazones. Interestingly, our synthetic protocol results in the formation of an aryldiazene radical, which undergoes 1,6-conjugate addition with p-QMs to furnish the arylhydrazones.
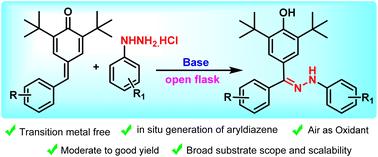
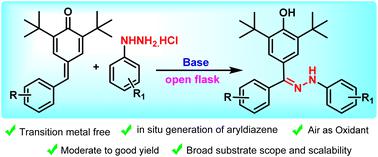
原位生成的芳基二氮烯与对醌甲酰胺的 1,6-共轭加成。
在此,我们报告了一种无过渡金属、以碱为介导的对醌甲酰胺(p-QMs)与芳基二氮烯的 1,6-共轭加成反应。在以空气为氧化剂的条件下,利用碱介导的方法,芳基肼被用于原位生成芳基二苯。芳基二氮烯通过自由基机制与对位醌甲酰胺发生 1,6 共轭加成反应,然后进行氧化重排,生成所需的产物--芳基肼。有趣的是,我们的合成方案会形成一个芳基二氮烯自由基,该自由基与对醌甲醚发生 1,6 共轭加成反应,生成芳基肼。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
The international home of synthetic, physical and biomolecular organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


