Topically applied novel TRPV1 receptor antagonist, ACD440 Gel, reduces evoked pain in healthy volunteers, a randomized, double-blind, placebo-controlled, crossover study
Abstract
Background
The TRPV1 receptor is a key molecule in pain generation. Previous development of oral TRPV1-antagonists was halted due to systemic heat insensitivity and body temperature alterations. The present Phase 1b study investigated the efficacy, safety and plasma exposure of a topically administered TRPV1-antagonist (ACD440 Gel) in healthy subjects.
Methods
The study comprised two parts.
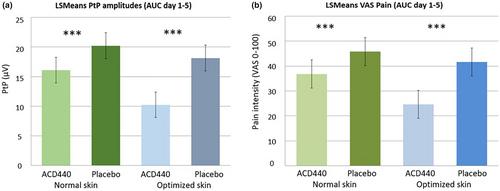
In part 1, 24 healthy subjects were included in this randomized double-blind, placebo-controlled, crossover trial. ACD440 Gel or Placebo was applied once daily and wiped off after 1 h, for 5 consecutive days. Assessments were done in normal skin, skin optimized for penetration (by stripping and occlusive gel application) and UVB-irradiated skin. Pain induced by thermo-nociceptive CO2 laser impulses generated laser-evoked potentials (LEPs), with readouts of peak-to-peak (PtP) amplitude in vertex-EEG and pain assessments by VAS (0–100). Endpoints include effects at 1 hour post-dose, AUC(Days 1–5) and AUC(0–24, Day 4). In UVB-irradiated skin, also pain on pinprick and skin redness were assessed.
Part 2 explored the plasma pharmacokinetics of ACD440.
Results
ACD440 Gel reduced LEP PtP amplitude and VAS pain, p < 0.001, in all skin conditions, versus placebo. In UVB-irradiated skin, pinprick pain was also reduced, p = 0.047. Effects were significant after 1 h, maintaining for at least 9 h. There were no adverse events or drug-induced erythema. Plasma exposures of ACD440 were too low to establish an elimination half-life of ACD400.
Conclusions
Topical ACD440 Gel demonstrated a significant analgesic effect on LEP, VAS score and pinprick pain, with low systemic exposures, supporting further clinical development.
Significance
This study demonstrates that the topical administration of a TRPV1-antagonist, ACD440 Gel, has potential as a new treatment for painful conditions affecting the skin, such as chronic peripheral neuropathic pain, without any local or systemic side effects.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


