Combination p53 activation and BCL-xL/BCL-2 inhibition as a therapeutic strategy in high-risk and relapsed acute lymphoblastic leukemia
IF 12.8
1区 医学
Q1 HEMATOLOGY
引用次数: 0
Abstract
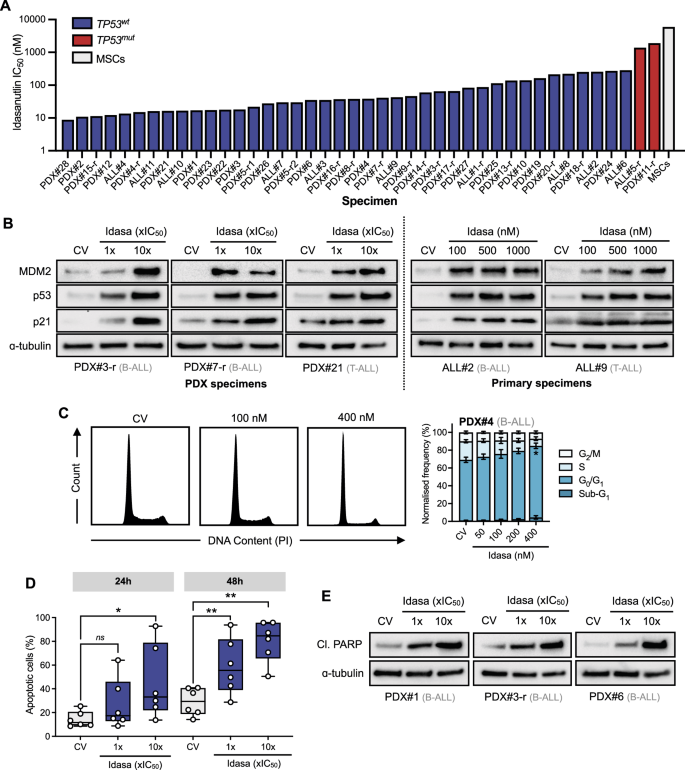
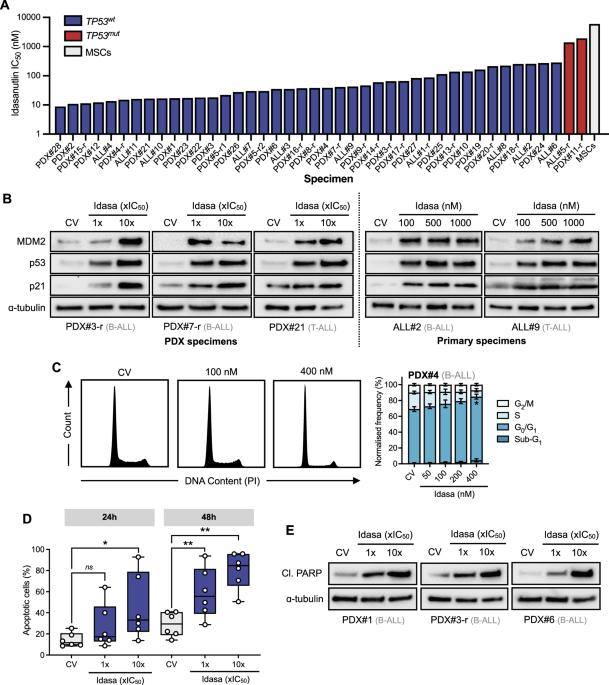
Due to the rarity of TP53 mutations in acute lymphoblastic leukemia (ALL), p53 re-activation by antagonism of the p53-MDM2 interaction represents a potential therapeutic strategy for the majority of ALL. Here, we demonstrate the potent antileukemic activity of the MDM2 antagonist idasanutlin in high-risk and relapsed ex vivo coculture models of TP53 wildtype ALL (n = 40). Insufficient clinical responses to monotherapy MDM2 inhibitors in other cancers prompted us to explore optimal drugs for combination therapy. Utilizing high-throughput combination screening of 1971 FDA-approved and clinically advanced compounds, we identified BCL-xL/BCL-2 inhibitor navitoclax as the most promising idasanutlin combination partner. The idasanutlin-navitoclax combination was synergistically lethal to prognostically-poor, primary-derived and primary patient blasts in ex vivo coculture, and reduced leukemia burden in two very high-risk ALL xenograft models at drug concentrations safely attained in patients; in fact, the navitoclax plasma concentrations were equivalent to those attained in contemporary “low-dose” navitoclax clinical trials. We demonstrate a preferential engagement of cell death over G1 cell cycle arrest, mechanistically implicating MCL-1-binding pro-apoptotic sensitizer NOXA. The proposed combination of two clinical-stage compounds independently under clinical evaluation for ALL is of high clinical relevance and warrants consideration for the treatment of patients with high-risk and relapsed ALL.
将 p53 激活与 BCL-xL/BCL-2 抑制相结合,作为高风险和复发急性淋巴细胞白血病的治疗策略
由于急性淋巴细胞白血病(ALL)中 TP53 突变的罕见性,通过拮抗 p53-MDM2 相互作用来重新激活 p53 是治疗大多数 ALL 的一种潜在策略。在这里,我们展示了MDM2拮抗剂idasanutlin在高危和复发的TP53野生型ALL体外细胞培养模型(n = 40)中的强效抗白血病活性。单药MDM2抑制剂在其他癌症中的临床反应不佳,促使我们探索联合疗法的最佳药物。通过对1971种FDA批准的临床先进化合物进行高通量联合筛选,我们发现BCL-xL/BCL-2抑制剂navitoclax是最有希望的idasanutlin联合搭档。在体外细胞培养中,idasanutlin-navitoclax 组合对预后较差的原发性和原发性患者胚泡具有协同致死作用,并能降低两种极高风险 ALL 异种移植模型中的白血病负担,其药物浓度在患者体内可安全达到;事实上,navitoclax 的血浆浓度与当代 "低剂量 "navitoclax 临床试验中达到的浓度相当。我们证明,细胞死亡优先于 G1 细胞周期停滞,从机理上讲,这与 MCL-1 结合促凋亡敏化剂 NOXA 有关。目前正在对这两种处于临床阶段的化合物进行独立的ALL临床评估,它们的联合用药具有很高的临床意义,值得考虑用于高危和复发ALL患者的治疗。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


