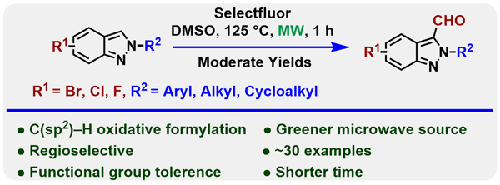
Regioselective C3-Formylation of 2H-Indazoles Using Selectfluor under Microwave-Assisted Conditions
IF 2.1
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
An efficient microwave-assisted Selectfluor-mediated regioselective C3-formylation of 2H-indazoles bearing a variety of alkyl and aryl substituents using DMSO as the formylating agent has been developed. This methodology provides access to 3-formyl 2H-indazoles with moderate to excellent yields. These functionalized indazoles are potentially useful as templates for drug discovery. Control experimental results suggest that this formylation probably proceeds through a radical pathway.
微波辅助条件下使用 Selectfluor 对 2H-Indazoles 进行区域选择性 C3-甲酰化反应
本研究开发了一种高效的微波辅助选择氟介导的区域选择性 C3-吲唑甲酰化反应,该反应以二甲基亚砜为甲酰化剂,含有多种烷基和芳基取代基。这种方法能以中等到极好的收率获得 3-甲酰基 2H-indazoles 。这些官能化的吲唑有可能成为药物研发的模板。对照实验结果表明,这种甲酰化可能是通过自由基途径进行的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


