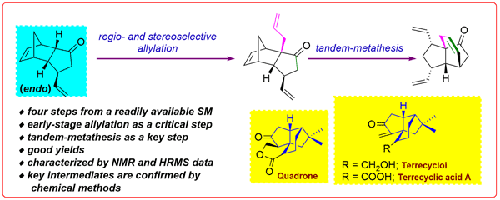
Synthesis of Propellane-Type 5/5/6-Tricyclic System via Tandem-Metathesis: A New Approach to Quadranoid Skeleton
IF 2.1
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
We disclose a useful synthetic study to prepare a propellane-type 5/5/6-tricyclic system which is present in diquinane-based natural products such as quadrone, terrecyclic acid A, terrecyclol, etc. The present methodology involves LDA-mediated regio- and stereoselective allylation, and tandem-metathesis as key steps. The target molecules were assembled in just two steps starting from a readily available building block, 3β-vinyl-tricyclic ketone, which is prepared from endo-dicyclopentadiene-1-one. All the compounds prepared here are characterized by NMR data analysis and/or chemical methods. The synthetic studies demonstrated here are useful in the synthesis of quadronoid type of natural products.
通过串联甲基化反应合成丙烷型 5/5/6 三环系统:四面体骨架的新方法
我们披露了一项有用的合成研究,用于制备丙烷型 5/5/6 三环体系,该体系存在于 quadrone、terrecyclic acid A、terrecyclol 等以二奎宁烷为基础的天然产物中。本方法的关键步骤包括 LDA 介导的区域和立体选择性烯丙基化和串联甲基化。目标分子只需两步就能组装完成,其起始原料是由内双环戊二烯-1-酮制备的 3β-vinyl-tricyclic ketone(3β-乙烯基三环酮)。本文制备的所有化合物都通过核磁共振数据分析和/或化学方法进行了表征。本文所展示的合成研究对合成四环类天然产物很有帮助。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


